Abstract
Introduction
Pain in Parkinson’s disease is poorly understood, and most patients with pain do not respond to dopaminergic drugs. We aimed to explore the mechanisms of dopa-responsive and -unresponsive pain by comparing such patients against patients without pain in terms of neural activity and functional connectivity in the brain.
Methods
We prospectively examined 31 Parkinson’s patients with dopa-responsive pain, 51 with dopa-unresponsive pain and 93 without pain using resting-state functional magnetic resonance imaging. Neural activity was assessed in terms of the amplitude of low-frequency fluctuation, while functional connectivity was assessed based on analysis of regions of interest.
Results
Patients with dopa-unresponsive pain showed significantly higher amplitude of low-frequency fluctuation in the right parahippocampal/lingual region than patients with no pain. However, there was no amplitude difference between the dopa-responsive pain group and the no pain group. Patients with dopa-unresponsive pain also differed significantly from patients with no pain in their functional connections between the superior temporal gyrus and other areas of cerebral cortex, between amygdala and thalamus and between the amygdala and putamen. Patients with dopa-responsive pain differed significantly from patients with no pain in their functional connections between temporal fusiform cortex and cerebellum, between precentral gyrus and temporal fusiform cortex and between precentral gyrus and cerebellum.
Conclusions
Regional neural activity and functional connectivity in the brain differ substantially among Parkinson’s patients with dopa-unresponsive pain, dopa-responsive pain or no pain. Our results suggest that dopa-responsive and -unresponsive pain may arise through different mechanisms, which may help guide the development of targeted therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. Dopa-unresponsive pain in Parkinson’s disease is associated with significantly higher neural activity in the right parahippocampal/lingual region. |
2. Patients with dopa-responsive pain show similar regional neural activity as patients without pain. |
3. Dopa-unresponsive pain in Parkinson’s appears to involve different alterations in functional connectivity than dopa-responsive pain. |
Introduction
Pain is a common non-motor symptom of Parkinson’s disease (PD) occurring in 40–85% of patients, and its incidence increases with disease progression [1, 2]. Pain influences quality of life more than motor symptoms [2]. Poor understanding of how pain arises in PD means that it is usually treated inadequately. While pain in some patients can be significantly alleviated through dopaminergic medication [3, 4], many patients do not respond to such therapy.
Neuroimaging studies make clear that PD involves alterations in pain perception and processing. In a positive emission tomography study, pain-free PD patients showed greater activation than healthy controls in ipsilateral insular and prefrontal cortex as well as contralateral anterior cingulate cortex in response to noxious cold stimuli [5]. Conversely, early untreated PD patients without pain showed significantly lower activation of the superior temporal gyrus (STG) and insula than controls based on functional magnetic resonance imaging (fMRI) [6]. In addition, patients subjected to heat stimulus also showed reduced connectivity between the basal ganglia and the salience network, mainly in the bilateral insulae and anterior cingulate gyri [7]. A resting-state fMRI study found that persistent pain in PD was associated with lower activity in the left frontal inferior orbital, greater bilateral activity in the cerebellum and right inferior temporal area as well as disconnection between the accumbens and hippocampus [8]. In contrast, patients with persistent pain did not show alterations in white matter or subcortex compared to patients without persistent pain.
We hypothesized that the supraspinal mechanisms for generating and maintaining pain in PD may differ between dopa-responsive and -unresponsive pain. To test this hypothesis, we used resting-state fMRI to explore the brain regions affected by each type of pain, based on comparisons with patients without pain.
Methods
Participants
In this prospective study, we recruited 236 patients at the Department of Neurology of Henan Provincial People’s Hospital (Zhengzhou, China) between February 2019 and January 2020 based on the following inclusion criteria: (1) clinically established PD according to the Movement Disorder Society Clinical Diagnostic Criteria for PD [9]; (2) no history of PD in first-degree relatives; (3) no MRI evidence of structural lesions related to other neurological disorders; (4) no head movement artifacts during MRI; (5) no dementia based on the Mini-Mental State Examination, after adjusting for age and education level [10].
Patients were excluded if they presented any of the following: (1) pain before PD onset; (2) pain that could be attributed to causes other than PD, such as Cox and gonarthrosis, orthopedic shoulder pain, spinal arthropathy osteoporosis, rheumatic immune disease, malignant tumor, infection or diabetes; (3) previous history of nervous system surgery; (4) severe psychosis or psychological diseases that might hinder pain perception or self-report; or (5) pain that lasted < 1 month.
This study was approved by the Ethics Committee of Henan Provincial People's Hospital, and procedures were performed according to the Declaration of Helsinki. Written informed consent was obtained from each individual.
Clinical Assessment
Patients underwent clinical assessments and fMRI during the on-medication phase. Examining patients by MRI during the off-medication phase is extremely challenging because their motor symptoms, especially tremor, are aggravated, which interferes with fMRI scanning. Another advantage of studying patients while on medication is that the potential confounding effects of depression, anxiety and cognitive impairment may be reduced [11].
PD severity was assessed using Part III of the Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS-III) [12]. Anxiety and depression were assessed using, respectively, the Hamilton Anxiety Scale (HAMA) [13] and Hamilton Depression Scale (HAMD) [14]. Pain was measured solely according to the patient’s subjective assessment [3, 4]. Patients were divided into those who reported no pain, those who had dopa-responsive pain if they reported that their pain was alleviated by dopaminergic medication or those with dopa-unresponsive pain if they reported no alleviation of pain with dopaminergic medication. Patients were asked to recall pain severity during the previous month using a visual analogue scale (VAS) [15]; patients with dopa-responsive pain were also asked to recall their maximal VAS score during the off-medication phase.
Resting-state fMRI
Images were acquired using a Siemens MAGNETOM Prisma 3-T scanner with a 64-channel head coil. Patients were asked to lie still, relax and keep their eyes open throughout the scanning. Functional images were obtained using axial echo-planar imaging with the following parameters: TR = 2000 ms, TE = 35 ms, flip angle = 80°, FOV = 240 × 240 mm, matrix size = 94 × 94, voxel dimensions 2.20 × 2.20 × 2.20 mm, slice thickness = 2.2 mm, number of slices = 75 and number of time points = 180.
Images were preprocessed and analyzed using Statistical Parametric Mapping version 12b (SPM12b; www.fil.ion.ucl.ac.uk/spm) and the CONN functional connectivity toolbox version 18_b [16] (http://www.nitrc.org/projects/conn). Images were preprocessed through the following steps [16, 17]: (1) functional slice-timing correction, (2) functional realignment and unwarping (subject motion estimation and correction), (3) functional outlier detection (artifact detection to identify outlier scans for scrubbing, www.nitrc.org/projects/artifact_detect/), (4) structural centering to (0,0,0) (translation), (5) functional direct normalization based on the Montreal Neurological Institute space and (8) functional smoothing (spatial convolution with a Gaussian kernel). Functional images were resliced at a resolution of 2 × 2 × 2 mm3 and smoothed using a Gaussian kernel (full width at half maximum, 8 mm). Subjects were excluded if their head motion exceeded 2 mm in displacement or 2° in rotation. In the denoising step, white matter, cerebrospinal fluid and head motion outliers were regressed. Low-frequency drift and high-frequency physiological noise were removed using bandpass filtering (0.01 < frequency < 0.08 Hz), while systematic shifts were removed using detrending.
First-level analysis of the CONN pipeline was conducted to generate individual amplitude of low-frequency fluctuation (ALFF) maps to evaluate regional neural activity [18]. Data were standardized across subjects by dividing the ALFF of each voxel by the global mean ALFF for all patients using the DPABI toolbox version 4.0 [19].
To evaluate functional connectivity in the brain, we analyzed activity among 132 regions of interest (ROIs), comprising 91 cortical and 15 subcortical ROIs from the FSL Harvard-Oxford Atlas [20], as well as 26 cerebellar ROIs from the Anatomical Automatic Labeling Atlas [21]. We defined 132 ROIs to take full advantage of the data available in the FSL Harvard-Oxford Atlas and Anatomical Automatic Labeling Atlas, thereby reducing the possibility that we might miss important differences. Potential correlations were obtained by applying a general linear model and bivariate correlation analysis weighted according to the hemodynamic response function based on first-level analysis of the CONN pipeline [16].
Statistical Analysis
Differences in clinicodemographic characteristics across the three groups were assessed for significance using one-way analysis of variance. Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) for Windows (version 22.0; SPSS, Chicago, IL, USA). Differences were considered significant if they were associated with p < 0.05.
To evaluate differences in regional neural activity between the no pain group and each of the other two groups, we analyzed ALFF from the individual standardized ALFF maps using the software package SPM12b. Age, sex and PD duration were entered as covariates to exclude their potential influence on ALFF. The significance threshold was defined as an uncorrected p = 0.001 at the initial voxel level and as a false discovery rate-adjusted p = 0.05 at the cluster level to correct for multiple comparisons. Pearson correlation analysis was performed to explore potential relationships between neuroimaging findings and clinical characteristics.
To evaluate changes in ROI-to-ROI functional connectivity between the no pain group and each of the other two groups, differences from the second-level analysis of the CONN pipeline were assessed using two-samples t tests [16]. The significance threshold was defined as a false discovery rate-adjusted p = 0.05 at the seed level to correct for multiple comparisons.
Results
Clinicodemographic Features
Of the 236 patients initially recruited, 61 were excluded and 175 were included in the final analysis, comprising 31 with dopa-responsive pain, 51 with dopa-unresponsive pain and 93 with no pain. PD patients had a long mean PD duration (6.6 ± 4.2 years). The three groups did not differ significantly in age, sex, age at PD onset, disease duration or UPDRS Part III score (Table 1). None of the patients reported using painkillers. Two patients reported using the antidepressants, one used sertraline, while the other used paroxetine.
The three groups differed significantly in HAMD or HAMA scores (Table 1). The dopa-responsive pain group reported a high VAS score of 6.0 ± 1.8 without medication and VAS score of 2.3 ± 2.0 during medication. Among those patients, 16 reported pain in the limbs; 8, pain in the trunk; and 7, pain in the limbs and trunk. The dopa-unresponsive pain group reported a VAS score of 4.1 ± 1.9. Among those patients, 23 reported pain in the limbs; 20, pain in the trunk; and 8, pain in the limbs and trunk.
Regional Spontaneous Brain Activity
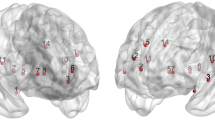
After adjusting for the effects of age, sex and disease duration, the dopa-unresponsive pain group showed significantly higher ALFF in the right parahippocampal/lingual gyrus than the no pain group [Montreal Neurological Institute coordinates (18 –34 –8); cluster size, 167 voxels; peak t value, 4.838; Fig. 1A]. No significant ALFF differences were identified between the dopa-responsive pain group and the no pain group. Among patients with dopa-unresponsive pain, ALFF in the right parahippocampal/lingual gyrus positively correlated with VAS score (R = 0.321, P = 0.022; Fig. 1B).
a Resting-state fMRI image of the brain showing greater amplitude of low-frequency fluctuation (ALFF) in the dopa-unresponsive pain group than in the no pain group in the parahippocampal/lingual_R region. b Correlation between pain intensity on the visual analogue scale (VAS) among patients with dopa-unresponsive pain and amplitude of low-frequency fluctuation (ALFF) in their parahippocampal/lingual_R region. L left, MNI Montreal Neurological Institute, R right
Functional Connectivity
The dopa-responsive pain group showed significantly stronger resting-state functional connections than the no pain group between the left temporal fusiform cortex (TFusC_l) and vermis_1_2 and vermis_3, between the left precentral gyrus (PreCG_l) and vermis_4_5, and between the vermis_1_2 and right cerebellum_6 (Table 2, Fig. 2A). Conversely, the dopa-responsive pain group showed significantly weaker functional connections than the no pain group between PreCG_l and the TFusC_l and between PreCG_l and right temporal fusiform cortex (TFusC_r) (Table 2, Fig. 2A).
Functional connectivity analysis of PD patients. a Compared to the no pain group, the dopa-responsive pain group showed significantly stronger functional connections of the TFusC_l with vermis_1_2 and vermis_3, between the PreCG_l and vermis_4_5 and between vermis_1_2 and the right cerebellum_6. Conversely, the dopa-responsive pain group showed significantly weaker functional connections of the PreCG_l with the TFusC_l and TFusC_r (p-FDR < 0.05 at seed level). b Compared to the no pain group, the dopa-unresponsive pain group showed significantly stronger functional connections of the STG_l with Vermis_6, left OFusG_l and right SPL_r. Conversely, the dopa-unresponsive pain group showed significantly weaker functional connections of the STG_l with MTG_l, MTG_r, AG_r and SFG_r; of the right amygdala with the right thalamus, right putamen and left thalamus; between the right thalamus and ITG_r and between the ITG_r and STG_r (p-FDR < 0.05 at seed level). Red indicates enhanced function connection; blue, weakened functional connection. Abbreviations: AG angular gyrus, aITG anterior division of the inferior temporal gyrus, aSTG anterior division of the superior temporal gyrus, aTFusC anterior division of the temporal fusiform cortex, l left, OFusG occipital fusiform gyrus, pMTG posterior division of the middle temporal gyrus, PreCG precentral gyrus, pSTG posterior division of the superior temporal gyrus, r right, SFG, superior frontal gyrus, SPL superior parietal lobule, toITG temporo-occipital part of the inferior temporal gyrus
In contrast, the dopa-unresponsive pain group did not show significant connectivity differences from the no pain group among the abovementioned ROIs. Instead, the dopa-unresponsive pain group showed significantly stronger connections than the no pain group between the left superior temporal gyrus (STG_l) on one hand and Vermis_6, left occipital fusiform gyrus (OFusG_l) and right superior parietal lobule (SPL_r) on the other (Table 3, Fig. 2B). Conversely, the dopa-unresponsive pain group showed significantly weaker connections of the STG_l with the left middle temporal gyrus (MTG_l), right middle temporal gyrus (MTG_r), right angular gyrus (AG_r) and right superior frontal gyrus (SFG_r); of the right amygdala with the right thalamus, right putamen and left thalamus; between the right thalamus and the right inferior temporal gyrus (ITG_r); as well as between the ITG_r and right superior temporal gyrus (STG_r) (Table 3, Fig. 2B). Functional connectivity among the abovementioned ROIs did not differ significantly between the dopa-responsive pain group and the no pain group.
Discussion
Our resting-state fMRI study suggests that dopa-unresponsive pain in PD is associated with significantly higher ALFF in the parahippocampal/lingual_R region and that the ALFF in this region may be associated with the severity of dopa-unresponsive pain. In addition, dopa-unresponsive pain in PD may be associated with altered functional connectivity between the STG and other cortical areas, between the amygdala and thalamus and between the amygdala and putamen. Such connectivity changes were not observed in PD patients with dopa-responsive pain. These findings may help explain why the pain in many PD patients does not respond to dopaminergic treatment, and they may help guide efforts to individualize pain treatments in PD.
Our study found higher ALFF in the right parahippocampal gyrus in the dopa-unresponsive pain group than in the no pain group. Similarly, acute eye pain patients showed higher ALFF in the right parahippocampal gyrus than healthy controls [22], as did patients with chronic low back pain following painful movement and individuals experiencing pricking pain [23, 24]. The parahippocampal gyrus, part of the limbic system, plays a critical role in memory and emotions [25], and its activity correlates with negative emotions [26]. Negative emotions can cause new pain or exacerbate existing pain, while pain itself can cause negative emotions [27]. These studies support our findings that activity in the parahippocampal gyrus correlates with dopa-unresponsive pain.
We found higher spontaneous activity in the lingual gyrus adjacent to the right parahippocampal gyrus in patients with dopa-unresponsive pain than in those with no pain. Similarly, toothache patients showed notably higher ALFF in the right lingual gyrus than healthy controls [28], as did individuals experiencing other types of dental stimulation [29]. PD patients with persistent pain show greater neural activity in the inferior temporary areas of the right lingual gyrus than patients without pain [8], which is consistent with our findings. These results suggest that activity in the lingual gyrus is associated with pain in PD.
Taken together, our analyses of ALFF suggest that dopa-unresponsive pain in PD involves activation of right lingual gyrus and parahippocampal gyrus. In fact, we found a positive correlation between neural activity in the right parahippocampal/lingual gyrus and severity of dopa-unresponsive pain. Our findings are consistent with the idea that dopa-unresponsive pain arises through mechanisms similar to those of other types of pain, whereas dopa-responsive pain may arise through a different mechanism. Specifically, we found that patients with dopa-unresponsive pain, but not those with dopa-responsive pain, showed significantly altered functional connectivity between the STG and other areas of cerebral cortex, between the amygdala and thalamus, and between the amygdala and putamen.
The STG has largely been ignored in pain imaging studies, likely in part because the link between STG function and pain is not obvious. The STG has traditionally been linked to auditory processing, language and social cognition. However, subjecting PD patients to heat stimuli inhibits the STG, implicating the STG in the processing of pain-related unpleasantness [6]. Other work has linked the STG to memories of pain [30]. The amygdala, part of the limbic area, plays a key role in emotional responses, affective states and disorders such as learned fear, anxiety and depression. The amygdala has also emerged as an important brain center for the emotional-affective dimension of pain [31]. The putamen is involved in sensory components of pain-related processes, beyond its well-established role in motor processes [32]. The thalamus is an important structure that, in healthy individuals, mediates the sensory discriminative component of pain (lateral pain pathway), the affective-motivational component of pain (medial pain pathway) [33] and the thermal pain threshold [34]. The subthalamic nucleus is a key structure in pain perception and modulation, and deep brain stimulation of the nucleus can treat pain in PD [35]. Our dopa-unresponsive pain group showed significantly altered functional connections between the STG and other areas of the cerebral cortex, between the amygdala and thalamus and between the amygdala and putamen. Several previous studies of PD patients have also indicated abnormalities in pain-related networks in the brain. One study reported that PD patients with persistent pain showed disrupted connectivity between the accumbens and hippocampus, as well as lower activity in the left frontal inferior orbital and greater bilateral cerebellar activity [8]. Our failure to identify exactly these abnormalities in our patients may reflect differences in the patient samples and statistical methods. An fMRI study showed lower “betweenness centrality” in pain networks in off-medication PD patients than in healthy controls [36]. A study of patients with early-stage PD stimulated by laser with lower energy showed abnormal activation patterns in the central pain matrix [37]. Our finding that the dopa-responsive pain group did not show altered functional connectivity in these brain regions strongly suggests different mechanisms for dopa-responsive and -unresponsive pain in PD.
Loss of nigrostriatal dopamine cells in PD causes a gradient of striatal dopamine depletion, leading to imbalance between direct (facilitatory) and indirect (inhibitory) pathways in the basal ganglia, ultimately resulting in bradykinesia [38]. These pathological changes co-occur with compensatory alterations, such as an increase in activity in more anterior corticostriatal circuits as well as increased connectivity to cortical regions normally weakly connected to the basal ganglia [39]. Dopaminergic drugs can affect activity in the cerebral cortex, such as by increasing connectivity between the prefrontal cortex and the supplementary motor area [39], which correlates with improved finger-tapping speed in PD patients. Dopaminergic medication can also reduce activity in the precentral gyrus and cerebellum of PD patients [40], while L-dopa has been shown to activate the TFusC [41]. We found that the dopa-responsive pain group, but not the dopa-unresponsive pain group, showed significantly altered resting-state functional connections between the TFusC and cerebellum, between the PreCG and TFusC and between the PreCG and cerebellum. Thus, we hypothesize that dopa-responsive pain in PD is associated with abnormal functional connectivity involving these brain regions. This would in turn help explain why dopaminergic drugs are effective against such pain.
There are a number of limitations to our study. First, our limited sample prevented us from classifying PD patients into the five traditional categories based on type of pain: musculoskeletal, radicular or neuropathic, dystonia-related, akathitic discomfort or primary (central) parkinsonian pain [42]. Therefore, the generalizability of our findings to various PD subpopulations remains to be determined. Second, we cannot exclude that the pain in some of our patients might have had causes other than PD, even though we were careful to include patients whose pain began after PD onset or whose pain could clearly be attributed to other conditions. Third, we cannot exclude that some patients whom we classified as having dopa-unresponsive pain may have responded if higher doses of medication had been used. We consider this less likely, given that our patients had a long mean PD duration (6.6 ± 4.2 years), and their response or non-response to dopaminergic drugs was established over a long period of time, during which different doses may have been tested. Fourth, we were careful to conduct fMRI when our patients were in the “on” state to reduce confounding effects due to motor symptoms, depression, anxiety and cognitive impairment [11]. At the same time, such medication may have influenced our fMRI analysis. Indeed, it may explain the lack of significant differences in ALFF between patients with dopa-responsive pain and patients without pain. Nevertheless, our patients with dopa-responsive pain reported appreciable pain during medication (VAS score, 2.3 ± 2.0), and we still detected significant differences in functional connectivity compared to patients reporting no pain. Last, we highlight the fact that we did not compare brain structure between patient groups in our study, which future studies should examine.
Conclusions
Our study provides the first fMRI evidence of differences in regional neural activity and functional connectivity in the brain between Parkinson’s patients with dopa-unresponsive or -responsive pain and patients without such pain. Dopa-unresponsive pain appears to involve similar mechanisms such as chronic lower back pain and toothache; dopa-responsive pain appears to involve brain regions known to be affected by dopaminergic medication. Our results strongly suggest that the two types of PD-associated pain arise through different mechanisms, which may help guide the development of targeted therapies.
References
Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, Bottacchi E, Cannas A, Ceravolo G, Ceravolo R, Cicarelli G, Gaglio RM, Giglia RM, Iemolo F, Manfredi M, Meco G, Nicoletti A, Pederzoli M, Petrone A, Pisani A, Pontieri FE, Quatrale R, Ramat S, Scala R, Volpe G, Zappulla S, Bentivoglio AR, Stocchi F, Trianni G, Dotto PD, P. s. group,. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord. 2009;24:1641–9. https://doi.org/10.1002/mds.22643.
Silverdale MA, Kobylecki C, Kass-Iliyya L, Martinez-Martin P, Lawton M, Cotterill S, Chaudhuri KR, Morris H, Baig F, Williams N, Hubbard L, Hu MT, Grosset DG, U. K. P. s. P. S. Collaboration. A detailed clinical study of pain in 1957 participants with early/moderate Parkinson’s disease. Parkinsonism Relat Disord. 2018;56:27–32. https://doi.org/10.1016/j.parkreldis.2018.06.001.
Angelika Nebe GE. Pain intensity on and off levodopa in patients with Parkinson’s disease. Mov Disord. 2009;24:1233–7. https://doi.org/10.1002/mds.22546.
Surucu O, Baumann-Vogel H, Uhl M, Imbach LL, Baumann CR. Subthalamic deep brain stimulation versus best medical therapy for L-dopa responsive pain in Parkinson’s disease. Pain. 2013;154:1477–9. https://doi.org/10.1016/j.pain.2013.03.008.
Brefel-Courbon C, Payoux P, Thalamas C, Ory F, Quelven I, Chollet F, Montastruc JL, Rascol O. Effect of levodopa on pain threshold in Parkinson’s disease: a clinical and positron emission tomography study. Mov Disord. 2005;20:1557–63. https://doi.org/10.1002/mds.20629.
Tan Y, Tan J, Luo C, Cui W, He H, Bin Y, Deng J, Tan R, Tan W, Liu T, Zeng N, Xiao R, Yao D, Wang X. Altered brain activation in early drug-naive Parkinson’s disease during heat pain stimuli: an fMRI study. Parkinsons Dis. 2015;2015: 273019. https://doi.org/10.1155/2015/273019.
Tan Y, Tan J, Deng J, Cui W, He H, Yang F, Deng H, Xiao R, Huang Z, Zhang X, Tan R, Shen X, Liu T, Wang X, Yao D, Luo C. Alteration of Basal Ganglia and right frontoparietal network in early drug-naive parkinson’s disease during heat pain stimuli and resting state. Front Hum Neurosci. 2015;9:467. https://doi.org/10.3389/fnhum.2015.00467.
Polli A, Weis L, Biundo R, Thacker M, Turolla A, Koutsikos K, Chaudhuri KR, Antonini A. Anatomical and functional correlates of persistent pain in Parkinson’s disease. Mov Disord. 2016;31:1854–64. https://doi.org/10.1002/mds.26826.
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–601. https://doi.org/10.1002/mds.26424.
Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269:2386–91.
Storch A, Schneider CB, Wolz M, Sturwald Y, Nebe A, Odin P, Mahler A, Fuchs G, Jost WH, Chaudhuri KR, Koch R, Reichmann H, Ebersbach G. Nonmotor fluctuations in Parkinson disease: severity and correlation with motor complications. Neurology. 2013;80:800–9. https://doi.org/10.1212/WNL.0b013e318285c0ed.
C. G. Goetz, B. C. Tilley, S. R. Shaftman, G. T. Stebbins, S. Fahn, P. Martinez-Martin, W. Poewe, C. Sampaio, M. B. Stern, R. Dodel, B. Dubois, R. Holloway, J. Jankovic, J. Kulisevsky, A. E. Lang, A. Lees, S. Leurgans, P. A. LeWitt, D. Nyenhuis, C. W. Olanow, O. Rascol, A. Schrag, J. A. Teresi, J. J. van Hilten, N. LaPelle and U. R. T. F. Movement Disorder Society. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–70. https://doi.org/10.1002/mds.22340.
Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5. https://doi.org/10.1111/j.2044-8341.1959.tb00467.x.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. https://doi.org/10.1136/jnnp.23.1.56.
Donald PAM, Price D, Amir R, Barbara B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45–56. https://doi.org/10.1016/0304-3959(83)90126-4.
Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–41. https://doi.org/10.1089/brain.2012.0073.
Zheng JH, Sun WH, Ma JJ, Wang ZD, Chang QQ, Dong LR, Shi XX, Li MJ. Resting-state functional magnetic resonance imaging in patients with Parkinson’s disease with and without constipation: a prospective study. Clin Auton Res. 2022;32:51–8. https://doi.org/10.1007/s10286-022-00851-8.
Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, Castellanos FX, Biswal BB, Milham MP. The oscillating brain: complex and reliable. Neuroimage. 2010;49:1432–45. https://doi.org/10.1016/j.neuroimage.2009.09.037.
Karunanayaka PR, Lee EY, Lewis MM, Sen S, Eslinger PJ, Yang QX, Huang X. Default mode network differences between rigidity- and tremor-predominant Parkinson’s disease. Cortex. 2016;81:239–50. https://doi.org/10.1016/j.cortex.2016.04.021.
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. https://doi.org/10.1016/j.neuroimage.2006.01.021.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. https://doi.org/10.1006/nimg.2001.0978.
Pan ZM, Li HJ, Bao J, Jiang N, Yuan Q, Freeberg S, Zhu PW, Ye L, Ma MY, Huang X, Shao Y. Altered intrinsic brain activities in patients with acute eye pain using amplitude of low-frequency fluctuation: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2018;14:251–7. https://doi.org/10.2147/NDT.S150051.
Zhang B, Jung M, Tu Y, Gollub R, Lang C, Ortiz A, Park J, Wilson G, Gerber J, Mawla I, Chan ST, Wasan A, Edwards R, Lee J, Napadow V, Kaptchuk T, Rosen B, Kong J. Identifying brain regions associated with the neuropathology of chronic low back pain: a resting-state amplitude of low-frequency fluctuation study. Br J Anaesth. 2019;123:e303–11. https://doi.org/10.1016/j.bja.2019.02.021.
Veldhuijzen DS, Nemenov MI, Keaser M, Zhuo J, Gullapalli RP, Greenspan JD. Differential brain activation associated with laser-evoked burning and pricking pain: An event-related fMRI study. Pain. 2009;141:104–13. https://doi.org/10.1016/j.pain.2008.10.027.
Catani M, Dell’acqua F, Thiebaut de Schotten M. A revised limbic system model for memory, emotion and behaviour. Neurosci Biobehav Rev. 2013;37:1724–37. https://doi.org/10.1016/j.neubiorev.2013.07.001.
Chan E, Baumann O, Bellgrove MA, Mattingley JB. Negative emotional experiences during navigation enhance parahippocampal activity during recall of place information. J Cogn Neurosci. 2014;26:154–64. https://doi.org/10.1162/jocn_a_00468.
Wiech K, Tracey I. The influence of negative emotions on pain: behavioral effects and neural mechanisms. Neuroimage. 2009;47:987–94. https://doi.org/10.1016/j.neuroimage.2009.05.059.
Yang J, Li B, Yu QY, Ye L, Zhu PW, Shi WQ, Yuan Q, Min YL, He YL, Shao Y. Altered intrinsic brain activity in patients with toothaches using the amplitude of low-frequency fluctuations: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2019;15:283–91. https://doi.org/10.2147/NDT.S189962.
Ettlin MBDA, Keller T, Luechinger R, Jäncke L, Palla S, Barlow A, Gallo LM, Lutz K. Interindividual differences in the perception of dental stimulation and related brain activity. Eur J Oral Sci. 2009;117:27–33. https://doi.org/10.1111/j.1600-0722.2008.00590.x.
Houde F, Martel M, Coulombe-Leveque A, Harvey MP, Auclair V, Mathieu D, Whittingstall K, Goffaux P, Leonard G. Perturbing the activity of the superior temporal gyrus during pain encoding prevents the exaggeration of pain memories: A virtual lesion study using single-pulse transcranial magnetic stimulation. Neurobiol Learn Mem. 2020;169: 107174. https://doi.org/10.1016/j.nlm.2020.107174.
Neugebauer V. Amygdala pain mechanisms. Handb Exp Pharmacol. 2015;227:261–84. https://doi.org/10.1007/978-3-662-46450-2_13.
Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, McHaffie JG, Coghill RC. The contribution of the putamen to sensory aspects of pain: insights from structural connectivity and brain lesions. Brain. 2011;134:1987–2004. https://doi.org/10.1093/brain/awr117.
A. H. A. Che Badariah Ab Aziz,. The role of the thalamus in modulating pain. Malays J Med Sci. 2006;13:11–8.
Badran BW, Caulfield KA, Stomberg-Firestein S, Summers PM, Dowdle LT, Savoca M, Li X, Austelle CW, Short EB, Borckardt JJ, Spivak N, Bystritsky A, George MS. Sonication of the anterior thalamus with MRI-Guided transcranial focused ultrasound (tFUS) alters pain thresholds in healthy adults: A double-blind, sham-controlled study. Brain Stimul. 2020;13:1805–12. https://doi.org/10.1016/j.brs.2020.10.007.
Mostofi A, Morgante F, Edwards MJ, Brown P, Pereira EAC. Pain in Parkinson’s disease and the role of the subthalamic nucleus. Brain. 2021;144:1342–50. https://doi.org/10.1093/brain/awab001.
Engels G, McCoy B, Vlaar A, Theeuwes J, Weinstein H, Scherder E, Douw L. Clinical pain and functional network topology in Parkinson’s disease: a resting-state fMRI study. J Neural Transm (Vienna). 2018;125:1449–59. https://doi.org/10.1007/s00702-018-1916-y.
Petschow C, Scheef L, Paus S, Zimmermann N, Schild HH, Klockgether T, Boecker H. Central pain processing in early-stage Parkinson’s disease: a laser pain fMRI study. PLoS ONE. 2016;11: e0164607. https://doi.org/10.1371/journal.pone.0164607.
Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397:2284–303. https://doi.org/10.1016/S0140-6736(21)00218-X.
Michely J, Volz LJ, Barbe MT, Hoffstaedter F, Viswanathan S, Timmermann L, Eickhoff SB, Fink GR, Grefkes C. Dopaminergic modulation of motor network dynamics in Parkinson’s disease. Brain. 2015;138:664–78. https://doi.org/10.1093/brain/awu381.
Bradberry TJ, Metman LV, Contreras-Vidal JL, van den Munckhof P, Hosey LA, Thompson JL, Schulz GM, Lenz F, Pahwa R, Lyons KE, Braun AR. Common and unique responses to dopamine agonist therapy and deep brain stimulation in Parkinson’s disease: an H(2)(15)O PET study. Brain Stimul. 2012;5:605–15. https://doi.org/10.1016/j.brs.2011.09.002.
Kim N, Goel PK, Tivarus ME, Hillier A, Beversdorf DQ. Independent component analysis of the effect of L-dopa on fMRI of language processing. PLoS ONE. 2010;5: e11933. https://doi.org/10.1371/journal.pone.0011933.
Ford B. Pain in Parkinson’s disease. Mov Disord. 2010;25(Suppl 1):S98-103. https://doi.org/10.1002/mds.22716.
Acknowledgements
We thank all the study participants for their participation and contribution. We also thank hospital staff for their dedication and contribution to this project.
Funding
This study was supported by the Henan Province Medical Science and Technology Research Program (SBGJ202102035).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
JHZ and WHS: Conception, organization, and execution of the study; data collection and statistical analysis; drafting of the manuscript. ZDW, QQC, LRD, XXS and MJL: Execution of the study; data collection; manuscript review. JJM: Conception and organization of the study; manuscript review; responsibility for the overall content as guarantor.
Disclosures
Jin Hua Zheng, Wen Hua Sun, Jian Jun Ma, Zhi Dong Wang, Qing Qing Chang, Lin Rui Dong, Xiao Xue Shi and Ming Jian Li have nothing to disclose.
Compliance with Ethics Guidelines
This study was approved by the Ethics Committee of Henan Provincial People's Hospital. The study was conducted in accordance with the Declaration of Helsinki and its later amendments. Informed consent was obtained from all participants in the study.
Data Availability
The data that support the findings of the present study are available from the corresponding authors upon reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zheng, J.H., Sun, W.H., Ma, J.J. et al. Differences in Brain Activity Between Dopa-Responsive and -Unresponsive Pain in Parkinson’s Disease. Pain Ther 11, 959–970 (2022). https://doi.org/10.1007/s40122-022-00404-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-022-00404-x