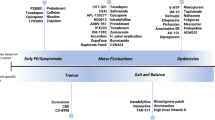
Abstract
After more than 40 years of clinical use, levodopa (LD) still remains the gold standard for symptomatic efficacy in Parkinson’s disease (PD). However, long-term treatment with LD is often complicated by the development of various types of motor response oscillations as well as drug-induced dyskinesias. These treatment-related motor complications evolve in approximately one-third of patients after only 2 years of LD exposure and, once established, they are difficult to treat and significantly contribute to overall disability and disease burden. Although first described soon after the introduction of LD, the pathophysiology of motor complications is still not completely understood. In fact, it is most likely that non-physiological pulsatile stimulation of dopamine receptors, which is followed by various downstream alterations, plays a key role in the development of LD-induced motor response oscillations and dyskinesias. This review outlines the various types of motor complications and will also address underlying mechanisms, treatment options, as well as impact on clinical disability and quality of life (QoL).


Similar content being viewed by others
References
Alexander G, Crutcher M (1990) Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13:266–271
Chapuis S, Ouchchane L, Metz O, Gerbaud L, Durif F (2005) Impact of the motor complications of Parkinson’s disease on the quality of life. Mov Disord 20:224–230
Clarke C, Davies P (2000) Systematic review of acute levodopa and apomorphine challenge tests in the diagnosis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 69:590–594
Colosimo C, Martínez-Martín P, Fabbrini G, Hauser R, Merello M, Miyasaki J, Poewe W, Sampaio C, Rascol O, Stebbins G, Schrag A, Goetz C (2010) Task force report on scales to assess dyskinesia in Parkinson’s disease: critique and recommendations. Mov Disord 25:1131–1142
Contin M, Riva R, Martinelli P, Cortelli P, Albani F, Baruzzi A (1999) Concentration-effect relationship of levodopa-benserazide dispersible formulation versus standard form in the treatment of complicated motor response fluctuation in Parkinson’s disease. Clin Neuropharmacol 22:351–355
Damiano A, McGrath M, Willian M, Snyder C, LeWitt P, Reyes P, Richter R, Means E (2000) Evaluation of a measurement strategy for Parkinson’s disease: assessing patient health-related quality of life. Qual Life Res 9:87–100
De Rijk M, Launer L, Berger K, Breteler M, Dartigues J, Baldereschi M, Fratiglioni L, Lobo A, Martinez-Lage J, Trenkwalder C, Hofman A (2000) Prevalence of Parkinson’s disease in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 54(Suppl 5):21–23
Djaldetti R, Baron J, Ziv I, Melamed E (1996) Gastric emptying in Parkinson’s disease: patients with and without response fluctuations. Neurology 46:1051–1054
Djaldetti R, Inzelberg R, Giladi N, Korczyn A, Peretz-Aharon Y, Rabey M, Herishano Y, Honigman S, Badarny S, Melamed E (2002) Oral solution of levodopa ethylester for treatment of response fluctuations in patients with advanced Parkinson’s disease. Mov Disord 17:297–302
Dodel R, Berger K, Oertel W (2001) Health-related quality of life and healthcare utilisation in patients with Parkinson’s disease. Pharmacoeconomics 19:1013–1038
Encarnacion E, Hauser R (2008) Levodopa-induced dyskinesias in Parkinson’s disease: etiology, impact on quality of life, and treatments. Eur Neurol 60:57–66
Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, Olanow C, Tanner C, Marek K, Group PS (2004) Levodopa and the progression of Parkinson’s disease. N Engl J Med 351:2498–2508
Forno L (1996) Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol 55:259–272
Gerfen C, Engber T, Mahan L et al (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250:1429–1432
Goetz C (1999) Rating scales for dyskinesias in Parkinson’s disease. Mov Disord 14(Suppl 1):48–53
Goetz C, Poewe W, Rascol O et al (2004) Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 19:1020–1028
PS Group (2000) A randomized controlled trial comparing pramipexole with levodopa in early Parkinson’s disease: design and methods of the CALM-PD Study. Clin Neuropharmacol 23:34–44
Hauser R, Friedlander J, Zesiewicz T, Adler C, Seeberger L, O’Brien C, Molho E, Factor S (2000) A home diary to assess functional status in patients with Parkinson’s disease with motor fluctuations and dyskinesia. Clin Neuropharmacol 23:75–81
Hauser R, McDermott M, Messing S (2006) Factors associated with the development of motor fluctuations and dyskinesias in Parkinson disease. Arch Neurol 63:1756–1760
Herrero M, Augood S, Hirsch E et al (1995) Effects of L-dopa on preproenkephalin and preprotachykinin gene expression in the MPTP-treated monkey striatum. Neuroscience 68:1189–1198
Hoehn M (1992) The natural history of Parkinson’s disease in the pre-levodopa and post-levodopa eras. Neurol Clin 10:311–339
Holloway R, Shoulson I, Fahn S, Kieburtz K, Lang A, Marek K, McDermott M, Seibyl J, Weiner W, Musch B, Kamp C, Welsh M, Shinaman A, Pahwa R, Barclay L, Hubble J, LeWitt P, Miyasaki J, Suchowersky O, Stacy M, Russell D, Ford B, Hammerstad J, Riley D, Standaert D, Wooten F, Factor S, Jankovic J, Atassi F, Kurlan R, Panisset M, Rajput A, Rodnitzky R, Shults C, Petsinger G, Waters C, Pfeiffer R, Biglan K, Borchert L, Montgomery A, Sutherland L, Weeks C, DeAngelis M, Sime E, Wood S, Pantella C, Harrigan M, Fussell B, Dillon S, Alexander-Brown B, Rainey P, Tennis M, Rost-Ruffner E, Brown D, Evans S, Berry D, Hall J, Shirley T, Dobson J, Fontaine D, Pfeiffer B, Brocht A, Bennett S, Daigneault S, Hodgeman K, O’Connell C, Ross T, Richard K, Watts A, Group PS (2004) Pramipexole vs. levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Arch Neurol 61:1044–1053
http://www.clinicaltrial.gov/ct2/show/NCT01071395 (2010) Validation of dyskinesia rating scales. In: clinicaltrials.gov (ed)
Hung S, Adeli G, Arenovich T, Fox S, Lang A (2007) Patient perception of dyskinesias in Parkinson’s disease. Neurology 68:A232
Jolkkonen J, Jenner P, Marsden C (1995) l-Dopa reverses altered gene expression of substance P but not enkephalin in the caudate-putamen of common marmosets treated with MPTP. Brain Res Mol Brain Res 32:297–307
Jost W (2010) Gastrointestinal dysfunction in Parkinson’s disease. J Neurol Sci 289:69–73
Karlsen K, Tandberg E, Arsland D, Larsen J (2000) Health related quality of life in Parkinson’s disease: a prospective longitudinal study. J Neurol Neurosurg Psychiatry 69:584–589
Kish S, Shannak K, Hornykiewicz O (1988) Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med 318:876–880
Kostic V, Przedborski S, Flaster E, Sternic N (1991) Early development of levodopa-induced dyskinesias and response fluctuations in young-onset Parkinson’s disease. Neurology 41:202–205
Krygowska-Wajs A, Lorens K, Thor P, Szczudlik A, Konturek S (2000) Gastric electromechanical dysfunction in Parkinson’s disease. Funct Neurol 15:41–46
Künzle H (1975) Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res 88:195–209
Leenders K, Poewe W, Palmer A, Brenton D, Frackowiak R (1986) Inhibition of L-[18F]fluorodopa uptake into human brain by amino acids demonstrated by positron emission tomography. Ann Neurol 20:258–262
Lees A (1987) A sustained-release formulation of l-dopa (Madopar HBS) in the treatment of nocturnal and early-morning disabilities in Parkinson’s disease. Eur Neurol 27:126–134
Lees A, Katzenschlager R, head J, Ben-Shlomo Y (2001) Ten year follow-up of three different initial treatments in de-novo PD: a randomized trial. Neurology 57:1687–1694
Luquin M, Scipioni O, Vaamonde J, Gershanik O, Obeso J (1992) Levodopa-induced dyskinesias in Parkinson’s disease: clinical and pharmacological classification. Mov Disord 7:117–124
Marconi R, Lefebvre-Caparros D, Bonnet A, Vidailhet M, Dubois B, Agid Y (1994) Levodopa-induced dyskinesias in Parkinson’s disease phenomenology and pathophysiology. Mov Disord 9:2–12
Marsden C (1994) Parkinson’s disease. J Neurol Neurosurg Psychiatry 57:672–681
Melamed E (1979) Early-morning dystonia. A late side effect of long-term levodopa therapy in Parkinson’s disease. Arch Neurol 36:308–310
Muenter M, Sharpless N, Tyce G, Darley F (1977) Patterns of dystonia (“I-D-I” and “D-I-D-”) in response to l-dopa therapy for Parkinson’s disease. Mayo Clin Proc 52:163–174
Nutt J (1990) Levodopa-induced dyskinesia: review, observations, and speculations. Neurology 40:340–345
Nutt J, Fellman J (1984) Pharmacokinetics of levodopa. Clin Neuropharmacol 7:35–49
Nutt J, Woodward W (1986) Levodopa pharmacokinetics and pharmacodynamics in fluctuating Parkinsonian patients. Neurology 36:739–744
Nutt J, Wooten G (2005) Clinical practice. Diagnosis and initial management of Parkinson’s disease. N Engl J Med 353:1021–1027
Oertel W, Wolters E, Sampaio C, Gimenez-Roldan S, Bergamasco B, Dujardin M, Grosset D, Arnold G, Leenders K, Hundemer H, Lledó A, Wood A, Frewer P, Schwarz J (2006) Pergolide versus levodopa monotherapy in early Parkinson’s disease patients: The PELMOPET Study. Mov Disord 21:343–353
Olanow C, Agid Y, Mizuno Y, Albanese A, Bonuccelli U, Bonucelli U, Damier P, De Yebenes J, Gershanik O, Guttman M, Grandas F, Hallett M, Hornykiewicz O, Jenner P, Katzenschlager R, Langston W, LeWitt P, Melamed E, Mena M, Michel P, Mytilineou C, Obeso J, Poewe W, Quinn N, Raisman-Vozari R, Rajput A, Rascol O, Sampaio C, Stocchi F (2004) Levodopa in the treatment of Parkinson’s disease: current controversies. Mov Disord 19:997–1005
Pacchetti C, Albani G, Martignoni E, Godi L, Alfonsi E, Nappi G (1995) “Off” painful dystonia in Parkinson’s disease treated with botulinum toxin. Mov Disord 10:333–336
Paci C, Thomas A, Onofrj M (2001) Amantadine for dyskinesia in patients affected by severe Parkinson’s disease. Neurol Sci 22:75–76
Pierantozzi M, Pietroiusti A, Brusa L, Galati S, Stefani A, Lunardi G, Fedele E, Sancesario G, Bernardi G, Bergamaschi A, Magrini A, Stanzione P, Galante A (2006) Helicobacter pylori eradication and l-dopa absorption in patients with PD and motor fluctuations. Neurology 66:1824–1829
Poewe W, Lees A, Stern G (1988) Dystonia in Parkinson’s disease: clinical and pharmacological features. Ann Neurol 23:73–78
Poewe W, Lees A, Stern G (1986) Low-dose l-dopa therapy in Parkinson’s disease: a 6-year follow-up study. Neurology 36:1528–1530
Quinn N, Critchley P, Marsden C (1987) Young onset Parkinson’s disease. Mov Disord 2:73–91
Rajput A (1992) Frequency and cause of Parkinson’s disease. Can J Neurol Sci 19(Suppl 1):103–107
Rajput A (2001) Levodopa prolongs life expectancy and is non-toxic to substantia nigra. Parkinsonism Relat Disord 8:95–100
Rascol O, Brooks D, Korczyn A, De Deyne P, Clarke C, Lang A (2000) A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. 056 Study Group. N Engl J Med 342:1484–1491
Rascol O, Brooks D, Melamed E, Oertel W, Poewe W, Stocchi F, Tolosa E, group Ls (2005) Rasagiline as an adjunct to levodopa in patients with Parkinson’s disease and motor fluctuations (LARGO, Lasting effect in Adjunct therapy with Rasagiline Given Once daily, study): a randomised, double-blind, parallel-group trial. Lancet 365:947–954 Mar 12–18
Rinne U, Bracco F, Chouza C, Dupont E, Gershanik O, Marti Masso J, Montastruc J, Marsden C (1998) Early treatment of Parkinson’s disease with cabergoline delays the onset of motor complications. Results of a double-blind levodopa controlled trial. The PKDS009 Study Group. Drugs 55(Suppl 1):23–30
Schrag A, Ben-Shlomo Y, Brown R, Marsden C, Quinn N (1998) Young-onset Parkinson’s disease revisited—clinical features, natural history, and mortality. Mov Disord 13:885–894
Schrag A, Quinn N (2000) Dyskinesias and motor fluctuations in Parkinson’s disease. A community-based study. Brain 123(Pt 11):2297–2305
Snow B, Macdonald L, Mcauley D, Wallis W (2000) The effect of amantadine on levodopa-induced dyskinesias in Parkinson’s disease: a double-blind, placebo-controlled study. Clin Neuropharmacol 23:82–85
Soykan I, Lin Z, Bennett J, McCallum R (1999) Gastric myoelectrical activity in patients with Parkinson’s disease: evidence of a primary gastric abnormality. Dig Dis Sci 44:927–931
Stacy M, Bowron A, Guttman M, Hauser R, Hughes K, Larsen J, LeWitt P, Oertel W, Quinn N, Sethi K, Stocchi F (2005) Identification of motor and nonmotor wearing-off in Parkinson’s disease: comparison of a patient questionnaire versus a clinician assessment. Mov Disord 20:726–733
Stocchi F, Rascol O, Kieburtz K, Poewe W, Jankovic J, Tolosa E, Barone P, Lang A, Olanow C (2010) Initiating levodopa/carbidopa therapy with and without entacapone in early Parkinson disease: the STRIDE-PD study. Ann Neurol 68:18–27
Tanner C, Goldman S (1996) Epidemiology of Parkinson’s disease. Neurol Clin 14:317–335
Verhagen Metman L, Del Dotto P, van den Munckhof P, Fang J, Mouradian M, Chase T (1998) Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson’s disease. Neurology 50:1323–1326
Whone A, Watts R, Stoessl A, Davis M, Reske S, Nahmias C, Lang A, Rascol O, Ribeiro M, Remy P, Poewe W, Hauser R, Brooks D, Group R-PS (2003) Slower progression of Parkinson’s disease with ropinirole versus levodopa: The REAL-PET Study. Ann Neurol 54:93–101
Witjas T, Kaphan E, Azulay J, Blin O, Ceccaldi M, Pouget J, Poncet M, Chérif A (2002) Nonmotor fluctuations in Parkinson’s disease: frequent and disabling. Neurology 59:408–413
Wolf E, Seppi K, Katzenschlager R, Hochschorner G, Ransmayr G, Schwingenschuh P, Ott E, Kloiber I, Haubenberger D, Auff E, Poewe W (2010) Long-term antidyskinetic efficacy of amantadine in Parkinson’s disease. Mov Disord 25(10):1357–1363
Zeng B, Pearce R, MacKenzie G, Jenner P (2000) Alterations in preproenkephalin and adenosine-2a receptor mRNA, but not preprotachykinin mRNA correlate with occurrence of dyskinesia in normal monkeys chronically treated with l-dopa. Eur J Neurosci 12:1096–1104
Conflict of interest
Klaus Seppi has received honoraria for speaking and consulting from Novartis, Boehringer Ingelheim, Lundbeck, Schwarz Pharma, UCB Pharma, and GlaxoSmithKline. Werner Poewe served as a consultant for Boehringer Ingelheim, Esai Ltd., Novartis, Sovay, and Teva. He was in the advisory panel for Boehringer Ingelheim, Esai Ltd., Genzyme, Novartis, Schering Plough, Sovay, Teva, and Valeant. He received research support from Boehringer Ingelheim and Astra Zeneca. He serves as a review editor for the Journal of Neurology and as a member of the editorial advisory board for the European Journal of Neurology. E. Hametner has no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hametner, E., Seppi, K. & Poewe, W. The clinical spectrum of levodopa-induced motor complications. J Neurol 257 (Suppl 2), 268–275 (2010). https://doi.org/10.1007/s00415-010-5719-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-010-5719-9