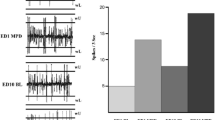
Abstract
Methylphenidate (MPD) is used to treat ADHD and as a cognitive enhancement and recreationally. MPD’s effects are not fully understood. One of the sites of psychostimulant action is the ventral tegmental area (VTA). The VTA neuronal activity was recorded from freely behaving rats using a wireless system. 51 animals were divided into groups: saline, 0.6, 2.5, and 10.0 mg/kg MPD. The same repetitive MPD dose can elicit either behavioral sensitization or tolerance; thus the evaluation of the VTA neuronal activity was based on the animals’ behavioral response to chronic MPD exposure: animals exhibiting behavioral tolerance or sensitization. Acute MPD elicits dose-related increases in behavioral activity. About half of the animals exhibited behavioral sensitization or tolerance to each of the MPD doses. 361 units were recorded from the VTA and exhibited similar spike shape on experimental day 1 (ED1) and on ED10. 71, 84, and 79 % of VTA units responded to acute 0.6, 2.5, and 10.0 mg/kg MPD, respectively. The neuronal baseline activity at ED10 was significantly modified in 94, 95, and 100 % of VTA units following 0.6, 2.5 and 10.0 mg/kg MPD, respectively. Following chronic MPD exposure, 91, 98, and 100 % exhibit either electrophysiological tolerance or sensitization of 0.6, 2.6, or 10.0 mg/kg MPD, respectively. In conclusion, the chronic administration of the same dose of MPD caused some animals to exhibit behavioral sensitization and other animals to exhibit tolerance. The VTA units recorded from animals exhibiting behavioral sensitization responded significantly differently to MPD from animals that exhibited behavioral tolerance.





Similar content being viewed by others
References
Algahim MF, Yang PB, Wilcox VT, Burau KD, Swann AC, Dafny N (2009) Prolonged methylphenidate treatment alters the behavioral diurnal activity pattern of adult male Sprague–Dawley rats. Pharmacol Biochem Behav 92:93–99
Arnsten AF, Dudley AG (2005) Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: relevance to therapeutic effects in attention deficit hyperactivity disorder. Behav Brain Funct 1:2
Askenasy EP, Taber KH, Yang PB, Dafny N (2007) Methylphenidate (Ritalin): behavioral studies in the rat. Int J Neurosci 117:757–794
Barron E, Yang PB, Swann AC, Dafny N (2009) Adolescent and adult male spontaneous hyperactive rats (SHR) respond differently to acute and chronic methylphenidate (Ritalin). Int J Neurosci 119:40–58
Beckstead RM, Domesick VB, Nauta WJ (1979) Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res 175:191–217
Bergheim M, Yang PB, Burau KD, Dafny N (2012) Adolescent rat circadian activity is modulated by psychostimulants. Brain Res 1431:35–45
Bonci A, Williams JT (1996) A common mechanism mediates long-term changes in synaptic transmission after chronic cocaine and morphine. Neuron 16:631–639
Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, Sarfatti SE, Vauss YC, Snell JW, Lange N, Kaysen D, Krain AL, Ritchie GF, Rajapakse JC, Rapoport JL (1996) Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry 53:607–616
Challman TD, Lipsky JJ (2000) Methylphenidate: its pharmacology and uses. Mayo Clin Proc 75:711–721
Chao J, Nestler EJ (2004) Molecular neurobiology of drug addiction. Annu Rev Med 55:113–132
Chong SL, Claussen CM, Dafny N (2012) Nucleus accumbens neuronal activity in freely behaving rats is modulated following acute and chronic methylphenidate administration. Brain Res Bull 87:445–456
Claussen CM, Dafny N (2012) Acute and chronic methylphenidate modulates the neuronal activity of the caudate nucleus recorded from freely behaving rats. Brain Res Bull 87:387–396
Dafny N (1975) Electrophysiological properties of caudate neurons following substantia nigra, motor cortex, and amygdaloid nuclear complex stimulation of the rat. Appl Neurophysiol 38:259–272
Dafny N (1980) Multiunit recording from medial basal hypothalamus following acute and chronic morphine treatment. Brain Res 190:584–592
Dafny N (1982) The hypothalamus exhibits electrophysiologic evidence for morphine tolerance and dependence. Exp Neurol 77:66–77
Dafny N, Terkel J (1990) Hypothalamic neuronal activity associated with onset of pseudopregnancy in the rat. Neuroendocrinology 51:459–467
Dafny N, Yang PB (2006) The role of age, genotype, sex, and route of acute and chronic administration of methylphenidate: a review of its locomotor effects. Brain Res Bull 68:393–405
Dietz DM, Dietz KC, Nestler EJ, Russo SJ (2009) Molecular mechanisms of psychostimulant-induced structural plasticity. Pharmacopsychiatry 42(Suppl 1):S69–S78
Fan D, Rich D, Holtzman T, Ruther P, Dalley JW, Lopez A, Rossi MA, Barter JW, Salas-Meza D, Herwik S, Holzhammer T, Morizio J, Yin HH (2011) A wireless multi-channel recording system for freely behaving mice and rats. PLoS ONE 6:e22033
Garland EJ (1998) Intranasal abuse of prescribed methylphenidate. J Am Acad Child Adolesc Psychiatry 37:1242–1243
Gatley SJ, Volkow ND, Gifford AN, Fowler JS, Dewey SL, Ding YS, Logan J (1999) Dopamine-transporter occupancy after intravenous doses of cocaine and methylphenidate in mice and humans. Psychopharmacology 146:93–100
Gaytan O, Ghelani D, Martin S, Swann A, Dafny N (1996) Dose response characteristics of methylphenidate on different indices of rats’ locomotor activity at the beginning of the dark cycle. Brain Res 727:13–21
Gaytan O, Ghelani D, Martin S, Swann A, Dafny N (1997a) Methylphenidate: diurnal effects on locomotor and stereotypic behavior in the rat. Brain Res 777:1–12
Gaytan O, Al-rahim S, Swann A, Dafny N (1997b) Sensitization to locomotor effects of methylphenidate in the rat. Life Sci 61:L101–L107
Gaytan O, Lewis C, Swann A, Dafny N (1999) Diurnal differences in amphetamine sensitization. Eur J Pharmacol 374:1–9
Gaytan O, Nason R, Alagugurusamy R, Swann A, Dafny N (2000) MK-801 blocks the development of sensitization to the locomotor effects of methylphenidate. Brain Res Bull 51:485–492
Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller D, Molina PE, Dewey SL (2000) Comparison between intraperitoneal and oral methylphenidate administration: a microdialysis and locomotor activity study. J Pharmacol Exp Ther 295:51–57
Grace AA (1991) Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience 41:1–24
Grace AA, Onn SP (1989) Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci 9:3463–3481
Gronier B (2011) In vivo electrophysiological effects of methylphenidate in the prefrontal cortex: involvement of dopamine D1 and alpha 2 adrenergic receptors. Eur Neuropsychopharmacol 21:192–204
Hyman SE, Malenka RC (2001) Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci 2:695–703
Izenwasser S, Coy AE, Ladenheim B, Loeloff RJ, Cadet JL, French D (1999) Chronic methylphenidate alters locomotor activity and dopamine transporters differently from cocaine. Eur J Pharmacol 373:187–193
Johnson SW, North RA (1992) Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol 450:455–468
Joyce MP, Rayport S (2000) Mesoaccumbens dopamine neuron synapses reconstructed in vitro are glutamatergic. Neuroscience 99:445–456
Kalivas PW, Duffy P (1993) Time course of extracellular dopamine and behavioral sensitization to cocaine, II. Dopamine perikarya. J Neurosci 13:276–284
Kalivas PW, Duffy P (1995) D1 receptors modulate glutamate transmission in the ventral tegmental area. J Neurosci 15:5379–5388
Kalivas PW, Stewart J (1991) Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev 16:223–244
Kalivas PW, Weber B (1988) Amphetamine injection into the ventral mesencephalon sensitizes rats to peripheral amphetamine and cocaine. J Pharmacol Exp Ther 245:1095–1102
Kalivas PW, Duffy P, DuMars LA, Skinner C (1988) Behavioral and neurochemical effects of acute and daily cocaine administration in rats. J Pharmacol Exp Ther 245:485–492
Kalivas PW, Sorg BA, Hooks MS (1993) The pharmacology and neural circuitry of sensitization to psychostimulants. Behav Pharmacol 4:315–334
Kalivas PW, Pierce RC, Cornish J, Sorg BA (1998) A role for sensitization in craving and relapse in cocaine addiction. J Psychopharmacol 12:49–53
Kallman WM, Isaac W (1975) The effects of age and illumination on the dose-response curves for three stimulants. Psychopharmacologia 40:313–318
Kauer JA (2004) Learning mechanisms in addiction: synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annu Rev Physiol 66:447–475
Kelz MB, Chen J, Carlezon WA Jr, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, Tkatch T, Baranauskas G, Surmeier DJ, Neve RL, Duman RS, Picciotto MR, Nestler EJ (1999) Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature 401:272–276
Kim Y, Teylan MA, Baron M, Sands A, Nairn AC, Greengard P (2009) Methylphenidate-induced dendritic spine formation and DeltaFosB expression in nucleus accumbens. Proc Natl Acad Sci USA 106:2915–2920
Lacroix D, Ferron A (1988) Electrophysiological effects of methylphenidate on the coeruleo-cortical noradrenergic system in the rat. Eur J Pharmacol 149:277–285
Lee MJ, Yang PB, Wilcox VT, Burau KD, Swann AC, Dafny N (2009) Does repetitive Ritalin injection produce long-term effects on SD female adolescent rats? Neuropharmacology 57:201–207
Lee SS, Humphreys KL, Flory K, Liu R, Glass K (2011) Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta-analytic review. Clin Psychol Rev 31:328–341
Lee SH, Seo WS, Sung HM, Choi TY, Kim SY, Choi SJ, Koo BH, Lee JH (2012) Effect of methylphenidate on sleep parameters in children with ADHD. Psychiatry Investig 9:384–390
Massello W III, Carpenter DA (1999) A fatality due to the intranasal abuse of methylphenidate (Ritalin). J Forensic Sci 44:220–221
Moratalla R, Vallejo M, Elibol B, Graybiel AM (1996) D1-class dopamine receptors influence cocaine-induced persistent expression of Fos-related proteins in striatum. NeuroReport 8:1–5
Morris JA, Gardner MJ (1988) Calculating confidence intervals for relative risks (odds ratios) and standardised ratios and rates. Br Med J (Clin Res Ed) 296:1313–1316
Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA (2008) Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience 152:1024–1031
Nestler EJ (2004) Molecular mechanisms of drug addiction. Neuropharmacology 47(Suppl 1):24–32
Nestler EJ (2005) Is there a common molecular pathway for addiction? Nat Neurosci 8:1445–1449
Nestler EJ (2008) Review. Transcriptional mechanisms of addiction: role of DeltaFosB. Philos. Trans R Soc Lond B Biol Sci 363:3245–3255
Papla I, Filip M, Przegalinski E (2002) Effect of intra-tegmental microinjections of 5-HT1B receptor ligands on the amphetamine-induced locomotor hyperactivity in rats. Pol J Pharmacol 54:351–357
Patrick KS, Markowitz JS (1997) Pharmacology of methylphenidate, amphetamine enantiomers and pemoline in attention-deficit hyperactivity disorder. Hum Psychopharmacol Clin Exp 12:527–546
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd edn. Academic Press, San Diego
Peakman MC, Colby C, Perrotti LI, Tekumalla P, Carle T, Ulery P, Chao J, Duman C, Steffen C, Monteggia L, Allen MR, Stock JL, Duman RS, McNeish JD, Barrot M, Self DW, Nestler EJ, Schaeffer E (2003) Inducible, brain region-specific expression of a dominant negative mutant of c-Jun in transgenic mice decreases sensitivity to cocaine. Brain Res 970:73–86
Pert A (1998) Neurobiological substrates underlying conditioned effects of cocaine. Adv Pharmacol 42:991–995
Perugini M, Vezina P (1994) Amphetamine administered to the ventral tegmental area sensitizes rats to the locomotor effects of nucleus accumbens amphetamine. J Pharmacol Exp Ther 270:690–696
Pierce RC, Kalivas PW (1995) Amphetamine produces sensitized increases in locomotion and extracellular dopamine preferentially in the nucleus accumbens shell of rats administered repeated cocaine. J Pharmacol Exp Ther 275:1019–1029
Pierce RC, Kalivas PW (1997) A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev 25:192–216
Podet A, Lee MJ, Swann AC, Dafny N (2010) Nucleus accumbens lesions modulate the effects of methylphenidate. Brain Res Bull 82:293–301
Prieto-Gomez B, Benitez MT, Vazquez-Alvarez AM, Yang PB, Reyes VC, Dafny N (2004) Dopaminergic ventral tegmental neurons modulated by methylphenidate. Life Sci 74:1581–1592
Prieto-Gomez B, Vazquez-Alvarez AM, Martinez-Pena JL, Reyes-Vazquez C, Yang PB, Dafny N (2005) Methylphenidate and amphetamine modulate differently the NMDA and AMPA glutamatergic transmission of dopaminergic neurons in the ventral tegmental area. Life Sci 77:635–649
Robinson TE (1984) Behavioral sensitization: characterization of enduring changes in rotational behavior produced by intermittent injections of amphetamine in male and female rats. Psychopharmacology 84:466–475
Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18:247–291
Robinson TE, Kolb B (1997) Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci 17:8491–8497
Robinson TE, Kolb B (1999) Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci 11:1598–1604
Robison AJ, Nestler EJ (2011) Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci 12:623–637
Salek RL, Claussen CM, Perez A, Dafny N (2012) Acute and chronic methylphenidate alters prefrontal cortex neuronal activity recorded from freely behaving rats. Eur J Pharmacol 679:60–67
Scheggi S, Leggio B, Masi F, Grappi S, Gambarana C, Nanni G, Rauggi R, De Montis MG (2002) Selective modifications in the nucleus accumbens of dopamine synaptic transmission in rats exposed to chronic stress. J Neurochem 83:895–903
Seeman P, Madras BK (1998) Anti-hyperactivity medication: methylphenidate and amphetamine. Mol Psychiatry 3:386–396
Seeman P, Madras B (2002) Methylphenidate elevates resting dopamine which lowers the impulse-triggered release of dopamine: a hypothesis. Behav Brain Res 130:79–83
Solanto MV (1998) Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res 94:127–152
Spanagel R, Herz A, Shippenberg TS (1992) Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci USA 89:2046–2050
Tang A, Wanchoo SJ, Swann AC, Dafny N (2009) Psychostimulant treatment for ADHD is modulated by prefrontal cortex manipulation. Brain Res Bull 80:353–358
Teo SK, Stirling DI, Thomas SD, Khetani VD (2003) Neurobehavioral effects of racemic threo-methylphenidate and its d and l enantiomers in rats. Pharmacol Biochem Behav 74:747–754
Vanderschuren LJ, Trezza V, Griffioen-Roose S, Schiepers OJ, Van LN, De Vries TJ, Schoffelmeer AN (2008) Methylphenidate disrupts social play behavior in adolescent rats. Neuropsychopharmacology 33:2946–2956
Vezina P (1993) Amphetamine injected into the ventral tegmental area sensitizes the nucleus accumbens dopaminergic response to systemic amphetamine: an in vivo microdialysis study in the rat. Brain Res 605:332–337
Volkow ND, Swanson JM (2003) Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry 160:1909–1918
Volkow ND, Swanson JM (2008) Does childhood treatment of ADHD with stimulant medication affect substance abuse in adulthood? Am J Psychiatry 165:553–555
Volkow ND, Fowler JS, Wang G, Ding Y, Gatley SJ (2002) Mechanism of action of methylphenidate: insights from PET imaging studies. J Atten Disord 6(Suppl 1):S31–S43
Volkow ND, Wang GJ, Fowler JS, Ding YS (2005) Imaging the effects of methylphenidate on brain dopamine: new model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol Psychiatry 57:1410–1415
Volz TJ, Farnsworth SJ, Hanson GR, Fleckenstein AE (2009) Method development and validation of an in vitro model of the effects of methylphenidate on membrane-associated synaptic vesicles. J Neurosci Methods 177:177–182
Wanchoo SJ, Lee MJ, Swann AC, Dafny N (2010) Bilateral six-hydroxydopamine administration to PFC prevents the expression of behavioral sensitization to methylphenidate. Brain Res 1312:89–100
Waterson P, Eason K, Tutt D, Dent M (2012) Using HIT to deliver integrated care for the frail elderly in the UK: current barriers and future challenges. Work 41(Suppl 1):4490–4493
White FJ, Kalivas PW (1998) Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend 51:141–153
White SR, Yadao CM (2000) Characterization of methylphenidate exposures reported to a regional poison control center. Arch Pediatr Adolesc Med 154:1199–1203
Wolf ME (1998) The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol 54:679–720
Yang PB, Amini B, Swann AC, Dafny N (2003) Strain differences in the behavioral responses of male rats to chronically administered methylphenidate. Brain Res 971:139–152
Yang PB, Swann AC, Dafny N (2006a) Sensory-evoked potentials recordings from the ventral tegmental area, nucleus accumbens, prefrontal cortex, and caudate nucleus and locomotor activity are modulated in dose-response characteristics by methylphenidate. Brain Res 1073–1074:164–174
Yang PB, Swann AC, Dafny N (2006b) Chronic methylphenidate modulates locomotor activity and sensory evoked responses in the VTA and NAc of freely behaving rats. Neuropharmacology 51:546–556
Yang PB, Swann AC, Dafny N (2006c) Dose-response characteristics of methylphenidate on locomotor behavior and on sensory evoked potentials recorded from the VTA, NAc, and PFC in freely behaving rats. Behav Brain Funct 2:3
Yang PB, Swann AC, Dafny N (2006d) Acute and chronic methylphenidate dose-response assessment on three adolescent male rat strains. Brain Res Bull 71:301–310
Yang PB, Swann AC, Dafny N (2007) Chronic administration of methylphenidate produces neurophysiological and behavioral sensitization. Brain Res 1145:66–80
Yang PB, Atkins KD, Dafny N (2011) Behavioral sensitization and cross-sensitization between methylphenidate amphetamine, and 3,4-methylenedioxymethamphetamine (MDMA) in female SD rats. Eur J Pharmacol 661:72–85
Acknowledgments
Supported by NIH DA RO1 027222 grant. We would also like to thank Catherine Claussen and Dr. Bin Tang for their help in carrying out this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jones, Z., Dafny, N. Acute and chronic dose–response effect of methylphenidate on ventral tegmental area neurons correlated with animal behavior. J Neural Transm 121, 327–345 (2014). https://doi.org/10.1007/s00702-013-1101-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-013-1101-2