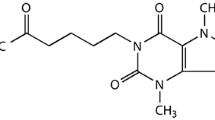
Abstract
Background
Subarachnoid hemorrhage (SAH) is a severe cerebrovascular disease frequently caused by ruptured aneurysms. Early brain injury (EBI) is the primary cause of morbidity and mortality in patients diagnosed with SAH and is associated with increased intracranial pressure, decreased cerebral blood flow and cerebral ischemia. Pentoxifylline (PTX) is a methylxanthine derivative clinically proven to improve perfusion in the peripheral microcirculation and has been shown to have neuroprotective effects in brain trauma and global cerebral ischemia in experimental animal models. This study aimed to determine the effect of PTX in experimental SAH, which has not been investigated yet.
Methods
An experimental SAH model was induced in male Wistar rats by autologous blood injection into the prechiasmatic cistern, and PTX was injected intraperitoneally immediately after SAH. The effects of PTX were evaluated 24 h after SAH via assessing the cerebral ultrastructure via transmission electron microscopy (TEM). Brain edema, blood-brain barrier (BBB) permeability, red blood cell deformability, tumor necrosis factor-alpha (TNF-alpha), nitrite-nitrate levels and apoptotic neuron death were also determined 24 h after SAH. The BBB permeability was measured by Evans blue (EB) extravasation, erythrocyte deformability was determined by filtration technique, and TNF-alpha and reactive nitrogen metobolites were analyzed in brain tissue by ELISA and spectral analysis, respectively. Apoptotic neurons were determined in brain sections by cleaved caspase-3 immunohistochemical analysis, and expression intensity was quantified using image J software.
Results
Cerebral ultrastructure in SAH group animals revealed intense perivascular edema and distortion in the astrocyte foot processes. PTX treatment attenuated structural deterioration due to SAH. Brain water content, BBB permeability, TNF-alpha, nitrite-nitrate levels and apoptotic neuronal death were significantly increased 24 h after SAH and were significantly alleviated by PTX treatment. There was no significant change in red cell deformability after SAH.
Conclusions
Our results show that PTX reduces brain edema, BBB permeability, TNF-alpha expression, reactive nitrogen metobolites and apopotosis in experimental SAH. Based on our findings we suggest that PTX exerts neuroprotection against SAH-induced EBI, which might be associated with the inhibition of inflammation and apoptotic neuronal cell death.






Similar content being viewed by others
References
Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ (2009) Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol 8:635–642
Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A (1994) Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 25:1342–1347
Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH (2013) Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res 4:432–446
Creager MA (2001) Medical management of peripheral arterial disease. Cardiol Rev 9:238–245
Windmeier C, Gressner AM (1997) Pharmacological aspects of pentoxifylline with emphasis on its inhibitory actions on hepatic fibrogenesis. Gen Pharmacol 29:181–196
Endres S, Fülle HJ, Sinha B, Stoll D, Dinarello CA, Gerzer R, Weber PC (1991) Cyclic nucleotides differentially regulate the synthesis of tumour necrosis factor-alpha and interleukin-1 beta by human mononuclear cells. Immunology 72:56–60
Lincoln TM, Cornwell TL (1993) Intracellular cyclic GMP receptor proteins. FASEB J 7:328–338
Sanz MJ, Alvarez A, Piqueras L, Cerdá M, Issekutz AC, Lobb RR, Cortijo J, Morcillo EJ (2002) Rolipram inhibits leukocyte-endothelial cell interactions in vivo through P- and E-selectin downregulation. Br J Pharmacol 135:1872–1881
Entzian P, Bitter-Suermann S, Burdon D, Ernst M, Schlaak M, Zabel P (1998) Differences in the anti-inflammatory effects of theophylline and pentoxifylline: important for the development of asthma therapy? Allergy 53:749–754
Kreth S, Ledderose C, Luchting B, Weis F, Thiel M (2010) Immunomodulatory properties of pentoxifylline are mediated via adenosine-dependent pathways. Shock 34:10–16
Toung TJ, Kirsch JR, Maruki Y, Traystman RJ (1994) Effects of pentoxifylline on cerebral blood flow, metabolism, and evoked response after total cerebral ischemia in dogs. Crit Care Med 22:273–281
Vakili A, Mojarrad S, Akhavan MM, Rashidy-Pour A (2011) Pentoxifylline attenuates TNF-α protein levels and brain edema following temporary focal cerebral ischemia in rats. Brain Res 1377:119–125
Eun BL, Liu XH, Barks JD (2000) Pentoxifylline attenuates hypoxic-ischemic brain injury in immature rats. Pediatr Res 47:73–78
Prunell GF, Mathiesen T, Svendgaard NA (2002) A new experimental model in rats for study of the pathophysiology of subarachnoid hemorrhage. Neuroreport 13:2553–2556
Vakili A, Zahedi khorasani M (2007) Post-ischemic treatment of pentoxifylline reduces cortical not striatal infarct volume in transient model of focal cerebral ischemia in rat. Brain Res 1144:186–191
Curek GD, Cort A, Yucel G, Demir N, Ozturk S, Elpek GO, Savas B, Aslan M (2010) Effect of astaxanthin on hepatocellular injury following ischemia/reperfusion. Toxicology 267:147–153
Shi SS, Zhang HB, Wang CH, Yang WZ, Liang RS, Chen Y, Tu XK (2015) Propofol attenuates early brain ınjury after subarachnoid hemorrhage in rats. J Mol Neurosci 57:538–545
Hardeman MR, Goedhart PT, Dobbe JGG, Lettings KP (1994) Laser Assisted optical rotational cell analyzer (LORCA). 1. A new instrument for measurement of various structural hemorheological parameters. Clin Hemorheol Microcirc 14:605–618
Baskurt OK, Meiselman HJ (2004) Analyzing shear stress-elongation index curves: comparison of two approaches to simplify data presentation. Clin Hemorheol Microcirc 31:23–30
van Gijn J, Rinkel GJ (2001) Subarachnoid haemorrhage: diagnosis, causes and management. Brain 124:249–278
Nishino A, Umegaki M, Fujinaka T, Yoshimine T (2010) Cilostazol attenuates cerebral vasospasm after experimental subarachnoid hemorrhage. Neurol Res 32:873–878
Pappas AC, Koide M, Wellman GC (2015) Astrocyte Ca2+ signaling drives ınversion of neurovascular coupling after subarachnoid hemorrhage. J Neurosci 35:13375–13384
Zhan Y, Krafft PR, Lekic T, Ma Q, Souvenir R, Zhang JH, Tang J (2015) Imatinib preserves blood–brain barrier integrity following experimental subarachnoid hemorrhage in rats. J Neurosci Res 93:94–103
Claassen J, Carhuapoma JR, Kreiter KT, Du EY, Connolly ES, Mayer SA (2002) Global cerebral edema after subarachnoid hemorrhage: frequency, predictors, and impact on outcome. Stroke 33:1225–1232
Dóczi T, Joó F, Adám G, Bozóky B, Szerdahelyi P (1986) Blood–brain barrier damage during the acute stage of subarachnoid hemorrhage, as exemplified by a new animal model. Neurosurgery 18:733–739
Orakcioglu B, Fiebach JB, Steiner T, Kollmar R, Jüttler E, Becker K, Schwab S, Heiland S, Meyding-Lamadé UK, Schellinger PD (2005) Evolution of early perihemorrhagic changes—ischemia vs. edema: an MRI study in rats. Exp Neurol 193:369–376
Jickling GC, Liu D, Ander BP, Stamova B, Zhan X, Sharp FR (2015) Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab 35:888–901
Lad SP, Hegen H, Gupta G, Deisenhammer F, Steinberg GK (2012) Proteomic biomarker discovery in cerebrospinal fluid for cerebral vasospasm following subarachnoid hemorrhage. J Stroke Cerebrovasc Dis 21:30–41
Gu C, Wang Y, Li J, Chen J, Yan F, Wu C, Chen G (2015) Rosiglitazone attenuates early brain injury after experimental subarachnoid hemorrhage in rats. Brain Res 1624:199–207
Yan J, Li L, Khatibi NH, Yang L, Wang K, Zhang W, Martin RD, Han J, Zhang J, Zhou C (2011) Blood–brain barrier disruption following subarchnoid hemorrhage may be faciliated through PUMA induction of endothelial cell apoptosis from the endoplasmic reticulum. Exp Neurol 230:240–247
Hasegawa T, Watanabe H, Ishii S (1980) Studies of intravascular components in cerebral vasospasm following subarachnoid hemorrhage. Neurosurg Rev 3:93–100
Baskurt OK, Meiselman HJ (2003) Blood rheology and hemodynamics. Semin Thromb Hemost 29:435–450
Chien S (1987) Red cell deformability and its relevance to blood flow. Annu Rev Physiol 49:177–192
Cicco G, Pirrelli A (1999) Red blood cell (RBC) deformability, RBC aggregability and tissue oxygenation in hypertension. Clin Hemorheol Microcirc 21:169–177
Altay O, Suzuki H, Hasegawa Y, Ostrowski RP, Tang J, Zhang JH (2014) Isoflurane on brain inflammation. Neurobiol Dis 62:365–371
Cahill J, Calvert JW, Zhang JH (2006) Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 26:1341–1353
Bernardo A, Ball C, Nolasco L, Moake JF, Dong JF (2004) Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood 104:100–106
Han J, Thompson P, Beutler B (1990) Dexamethasone and pentoxifylline inhibit endotoxin-induced cachectin/tumor necrosis factor synthesis at separate points in the signaling pathway. J Exp Med 172:391–394
Marcinkiewicz J, Grabowska A, Lauterbach R, Bobek M (2000) Differential effects of pentoxifylline, a non-specific phosphodiesterase inhibitor, on the production of IL-10, IL-12 p40 and p35 subunits by murine peritoneal macrophages. Immunopharmacology 49:335–343
Yang GY, Gong C, Qin Z, Liu XH, Lorris Betz A (1999) Tumor necrosis factor alpha expression produces increased blood–brain barrier permeability following temporary focal cerebral ischemia in mice. Brain Res Mol Brain Res 69:135–143
Sadamitsu D, Kuroda Y, Nagamitsu T, Tsuruta R, Inoue T, Ueda T, Nakashima K, Ito H, Maekawa T (2001) Cerebrospinal fluid and plasma concentrations of nitric oxide metabolites in postoperative patients with subarachnoid hemorrhage. Crit Care Med 29:77–79
Suzuki M, Asahara H, Endo S, Inada K, Doi M, Kuroda K, Ogawa A (1999) Increased levels of nitrite/nitrate in the cerebrospinal fluid of patients with subarachnoid hemorrhage. Neurosurg Rev 22:96–98
Kikuchi T, Okuda Y, Kaito N, Abe T (1995) Cytokine production in cerebrospinal fluid after subarachnoid haemorrhage. Neurol Res 17:106–108
Zheng B, Zheng T, Wang L, Chen X, Shi C, Zhao S (2010) Aminoguanidine inhibition of iNOS activity ameliorates cerebral vasospasm after subarachnoid hemorrhage in rabbits via restoration of dysfunctional endothelial cells. J Neurol Sci 295:97–103
Lauterbach R, Grabowska A, Marcinkiewicz J (1995) Effect of pentoxifylline on nitric oxide released by murine macrophages. Biol Neonate 67:72–76
Yang YQ, Li H, Zhang XS, Li W, Huang LT, Yu Z, Jiang TW, Chen Q, Hang CH (2015) Inhibition of SENP3 by lentivirus induces suppression of apoptosis in experimental subarachnoid hemorrhage in rats. Brain Res 1622:270–278
Cheng G, Wei L, Zhi-Dan S, Shi-Guang Z, Xiang-Zhen L (2009) Atorvastatin ameliorates cerebral vasospasm and early brain injury after subarachnoid hemorrhage and inhibits caspase-dependent apoptosis pathway. BMC Neurosci 10:7
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309–1312
Nadia Sharifi Z, Movassaghi S, Mohamadzadeh F, Soleimani Asl S, Pourheydar B, Mehdizadeh M (2015) Reduction in ischemic brain injury following the administration of pentoxifylline after transient global ischemia/reperfusion in a rat model. Med J Islam Repub Iran 29:193
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Akdeniz University Research Foundation provided financial support in the form of grant funding (no: TSA-2015-925). The sponsor had no role in the design or conduct of this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Goksu, E., Dogan, O., Ulker, P. et al. Pentoxifylline Alleviates Early Brain Injury in a Rat Model of Subarachnoid Hemorrhage. Acta Neurochir 158, 1721–1730 (2016). https://doi.org/10.1007/s00701-016-2866-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-016-2866-5