Abstract
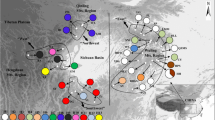
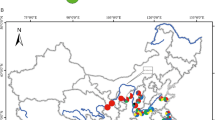
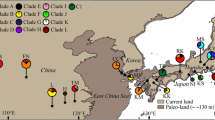
Past geological and climatic changes have promoted regional-scale intraspecific differentiation and range contraction/expansion in many temperate plants. However, little is known about how the desert species in central Asia responded to past geological and climatic changes, especially for a few widespread desert plants. In the present study, we aimed to survey the population structure and phylogeographical history of Allium mongolicum, which is widely distributed in the deserts of northwestern China. We sequenced two chloroplast DNA fragments (accD-psaI and psbA-trnH) for 418 individuals from 38 populations across the whole range of the species. Fourteen chlorotypes were identified, and three out of them were dominant. All populations were divided into three larger distinct groups by SAMOVA, which was largely congruent with the geographical division based on the Monmonier’s maximum-difference algorithm. Each of the groups occupied a distinct geographical region with a specific dominant chlorotype. Analysis of molecular variance showed that a high proportion of the total genetic variation (70.05%) existed among the three regions. The demographic dynamic tests indicated that the desert species had experienced a sudden regional-scale range expansion/recolonization in the Quaternary glaciers, which was further identified by the ecological niche modeling. These results suggest that the species has a distinct regional-scale differentiation as well as multiple geographically isolated refugia. Our results further enforce the idea that the environmental changes since the late Miocene greatly promoted differentiation of desert plants in northwestern China, and the Quaternary climatic oscillations played an important role in structuring the current populations of these species.



Similar content being viewed by others
References
Abbott RJ, Brochmann C (2003) History and evolution of the arctic flora: in the footsteps of Eric Hultén. Molec Ecol 12:299–313. doi:10.1046/j.1365-294X.2003.01731.x
Abbott RJ, Smith LC, Milne RI, Crawford RM, Wolff K, Balfour J (2000) Molecular analysis of plant migration and refugia in the Arctic. Science 289:1343–1346. doi:10.1126/science.289.5483.1343
Afzal-Rafii Z, Dodd A (2007) Chloroplast DNA supports a hypothesis of glacial refugia over postglacial recolonization in disjunct populations of black pine (Pinus nigra) in Western Europe. Molec Ecol 16:723–736. doi:10.1111/j.1365-294X.2006.03183.x
Al-Shehbaz IA, Beilstein MA, Kellogg EA (2006) Systematics and phylogeny of the Brassicaceae (Cruciferae): an overview. Pl Syst Evol 259:89–120. doi:10.1007/s00606-006-0415-z
Anderson LL, Hu FS, Nelson DM, Petit RJ, Paige KN (2006) Iceage endurance: DNA evidence of a white spruce regugium in Alaska. Proc Natl Acad Sci USA 103:12447–12450. doi:10.1073/pnas.0605310103
Avise JC (1987) Identification and interpretation of mitochondrial DNA stocks in marine species. In: Kumpf HE (ed) Proceedings of the stock identification workshop, November 5–7, 1985, Panama City Beach, Florida. National Marine Fisheries Service, Southeast Fisheries Center, Miami
Avise JC (2000) Phylogeography: the history and formation of species. Harvard University Press, London
Avise JC (2004) Molecular markers, natural history, and evolution. Inauer Associates, Sunderland
Bacles CFE, Lowe AJ, Ennos RA (2006) Effective seed dispersal across a fragmented landscape. Science 311:628–628. doi:10.1126/science.1121543
Bandelt HJ, Forster P, Röhl A (1999) Median-joining networks for inferring intraspecific phylogenies. Molec Biol Evol 16:37–48. doi:10.1371/journal.pone.0001538
Burban C, Petit RJ (2003) Phylogeography of maritime pine inferred with organelle markers having contrasted inheritance. Molec Ecol 12:1487–1495. doi:10.1046/j.1365-294X.2003.01817.x
Bush ABG, Little EC, Rokosh D, White D, Rutter NW (2004) Investigation of the spatio-temporal variability in Eurasian Late Quaternary loess-paleosol sequences using a coupled atmosphere-ocean general circulation model. Quaternary Sci Rev 23:481–498. doi:10.1016/j.quascirev.2003.08.009
Chen KM, Abbott RJ, Milne RI, Tian XM, Liu JQ (2008) Phylogeography of Pinus tabulaeformis Carr. (Pinaceae), a dominant species of coniferous forest in northern China. Molec Ecol 17:4276–4288. doi:10.1016/j.ypmed.2004.04.055
Comes HP, Kadereit JW (1998) The effect of Quaternary climatic changes on plant distribution and evolution. Trends Pl Sci 3:432–438. doi:10.1016/S1360-1385(98)01327-2
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf material. Phytochem Bull 19:11–15
Dumolin S, Demesure B, Petit RJ (1995) Inheritance of chloroplast and mitochondrial genomes in pedunculate oak investigated with an efficient PCR method. Theor Appl Genet 91:1253–1256. doi:10.1007/BF00220937
Dupanloup I, Schneider S, Excoffier L (2002) A simulated annealing approach to define the genetic structure of populations. Molec Ecol 11:2571–2581. doi:10.1046/j.1365-294X.2002.01650.x
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction sites. Genetics 131:479–491
Excoffier L, Laval G, Schneider S (2005) Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol Bioinform 1:47–50
Fawcett T (2006) An introduction to ROC analysis. Pattern Recogn Lett 27:861–874. doi:10.1016/j.patrec.2005.10.010
Fehlberg SD, Ranker TA (2009) Evolutionary history and phylogeography of Encelia farinosa (Asteraceae) from the Sonoran, Mojave, and Peninsular Deserts. Molec Phylogenet Evol 50:326–335. doi:10.1016/j.ympev.2008.11.011
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791. doi:10.2307/2408678
Felsenstein J (2005) PHYLIP (Phylogeny Inference Package) version 3.6. Distributed by the author. Department of Genome Sciences, University of Washington, Seattle
Fu YX (1997) Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147:915–925. doi:10.1371/journal.pone.0092293
Garrick R, Nason J, Meadows C, Dyer R (2009) Not just vicariance: phylogeography of a Sonoran Desert euphorb indicates a major role of range expansion along the Baja peninsula. Molec Ecol 18:1916–1931. doi:10.1111/j.1365-294X.2009.04148.x
Godbaut J, Jaramillo-Corea JP, Beaulieu J, Bousquet J (2005) A mitochondrial DNA minisatellite reveals the postglacial history of jack pine (Pinus banksiana), a broad-range North American conifer. Molec Ecol 14:3497–3512. doi:10.1111/j.1365-294X.2005.02674.x
Guo ZT, Ruddiman WF, Hao QZ, Wu HB, Qiao YS, Zhu RX, Peng SZ, Wei JJ, Yuan BY, Liu TS (2002) Onset of Asian desertification by 22 Myr ago inferred from loess deposits in China. Nature 416:159–163. doi:10.1038/416159a
Guo YP, Zhang R, Chen CY, Zhou DW, Liu JQ (2010) Allopatric divergence and regional range expansion of Juniperus sabina in China. J Syst Evol 48:153–160. doi:10.1111/j.1759-6831.2010.00073.x
Hamilton MB (1999) Four primer pairs for the amplification of chloroplast intergenic regions with intraspecific variation. Molec Ecol 8:521–523. doi:10.1046/j.1365-294X.1999.00510.x
Hamilton MB, Miller JR (2002) Comparing relative rates of pollen and seed gene flow in the island model using nuclear and organelle measures of population structure. Genetics 162:1897–1909
Hamper A, Arroyo J, Jordano P, Petit RJ (2003) Rangewide phylogeography of a bird-dispersed Eurasian shrub: contrasting Mediterranean and temperate glacial refugia. Molec Ecol 12:3415–3426. doi:10.1016/S0008-6223(00)00148-2
Harpending HC (1994) Signature of ancient population growth in a low-resolution mitochondrial DNA mismatch distribution. Hum Biol 66:591–600. doi:10.1371/journal.pone.0038184
Hasumi H, Emori S (2004) K-1 coupled GCM (MIROC) description. Center for Climate System Research, University of Tokyo, Tokyo
Heuertz M, Fineschi S, Anzidei M (2004) Chloroplast DNA variation and postglacial recolonization of common ash (Fraxinus excelsior L.) in Europe. Molec Ecol 13:3437–3452. doi:10.1007/s00606-009-0177-5
Hewitt GM (1996) Some genetic consequence of ice ages, and their role in divergence and speciation. Biol J Linn Soc 58:247–276. doi:10.1007/s10336-004-0024-y
Hewitt GM (2000) The genetic legacy of the Quaternary ice ages. Nature 405:907–913. doi:10.1038/35016000
Hewitt GM (2004) Genetic consequences of climatic oscillations in the Quaternary. Philos Trans R Soc Lond Ser B Biol Sci 359:183–195. doi:10.1098/rstb.2003.1388
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978. doi:10.1002/joc.1276
Hwang SY, Lin TP, Ma CS, Lin CL, Chung JD, Yang JC (2003) Postglacial population growth of Cunninghamia konishii (Cupressaceae) inferred from phylogeographical and mismatch analysis of chloroplast DNA variation. Molec Ecol 12:2689–2695. doi:10.1046/j.1365-294X.2003.01935.x
Jaramillo-Correa JP, Beaulieu J, Bousquet J (2004) Variation in mitochondrial DNA reveals multiple distant glacial refugia in black spruce (Picea mariana), a transcontinental North American conifer. Molec Ecol 13:2735–2747. doi:10.1111/j.1365-294X.2004.02258.x
Jia DR, Liu TL, Wang LY, Zhou DW, Liu JQ (2011) Evolutionary history of an alpine shrub Hippophae tibetana (Elaeagnaceae): allopatric divergence and regional expansion. Biol J Linn Soc 102:37–50. doi:10.1111/j.1095-8312.2010.01553.x
Kropf M, Kandereit JW, Comes HP (2003) Differential cycles of range contraction and expansion in European high mountain plants during the late Quaternary: insight from Pritzelago alpine (L.) O. Kuntze (Brassicaceae). Molec Ecol 12:931–949. doi:10.1046/j.1365-294X.2003.01781.x
Li ZH, Chen J, Zhao GF, Guo YP, Kou YX, Ma YZ, Wang G, Ma XF (2012) Response of a desert shrub to past geological and climatic change: a phylogeographic study of Reaumuria soongorica (Tamaricaceae) in western China. J Syst Evol 50:351–361. doi:10.1111/j.1759-6831.2012.00201.x
Liu CR, Berry PM, Dawson TP, Pearson RG (2005) Selecting thresholds of occurrence in the prediction of species distributions. Ecography 28:385–393. doi:10.1111/j.0906-7590.2005.03957.x
Liu YF, Wang Y, Huang HW (2009) Species-level phylogeographical history of Myricaria plants in the mountain ranges of western China and the origin of M. laxiflora in the three gorges mountain region. Molec Ecol 18:2700–2712. doi:10.1111/j.1365-294X.2009.04214.x
Lucarini V, Calmanti S, Dell’Aquila A, Ruti PM, Speranza A (2007) Intercomparison of the northern hemisphere winter midlatitude atmospheric variability of the IPCC models. Clim Dynam 28:829–848. doi:10.1007/s00382-006-0213-x
Manni F, Guérard E, Heyer E (2004) Geographic patterns of (genetic, morphologic, linguistic) variation: how barriers can be detected by using Monmonier’s algorithm. Hum Biol 76:173–190
Marquardt PE, Epperson BK (2004) Spatial and population genetic structure of microsatellites in white pine. Molec Ecol 13:3305–3315. doi:10.1111/j.1365-294X.2004.02341.x
Meng HH, Zhang ML (2011) Phylogeography of Lagochilus ilicifolius (Lamiaceae) in relation to Quaternary climatic oscillation and aridification in northern China. Biochem Syst Ecol 39:787–796. doi:10.1016/j.bse.2011.07.015
Nason JD, Hamrick J, Fleming TH (2002) Historical vicariance and postglacial colonization effects on the evolution of genetic structure in Lophocereus, a Sonoran Desert columnar cactus. Evolution 56:2214–2226. doi:10.1554/0014-3820(2002)056[2214:HVAPCE]2.0.CO;2
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Opgenoorth L, Vendramin GG, Mao KS, Miehe G, Miehe S, Liepelt S, Liu JQ, Ziegenhagen B (2010) Tree endurance on the Tibetan Plateau marks the world’s highest known tree line of the Last Glacial Maximum. New Phytol 185:332–342. doi:10.1111/j.1469-8137.2009.03007.x
Palmé AE, Semerikov V, Lascoux M (2003) Absence of geographical structure of chloroplast DNA variation in sallow, Salix caprea L. Heredity 91:465–474. doi:10.1038/sj.hdy.6800307
Petit RJ, Grivet D (2002) Optimal randomization strategies when testing the existence of a phylogeographic structure. Genetics 161:469–471
Petit RJ, Aguinagalde I, de Beaulieu JL, Bittkau C, Brewer S, Cheddadi R, Ennos R, Fineschi S, Grivet D, Lascoux M, Mohanty A, Muller-Starck G, Demesure-Musch B, Palme A, Martin JP, Rendell S, Vendramin GG (2003) Glacial refugia: hotspots but not melting pots of genetic diversity. Science 300:1563–1565. doi:10.1126/science.1083264
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Model 190:231–259. doi:10.1016/j.ecolmodel.2005.03.026
Pons O, Petit RJ (1996) Measuring and testing genetic differentiation with ordered versus unordered alleles. Genetics 144:1237–1245
Provan J, Bennett KD (2008) Phylogeographic insights into cryptic glacial refugia. Trends Ecol Evol 23:564–571. doi:10.1016/j.tree.2008.06.010
Qian H, Ricklefs RE (2000) Large-scale processes and the Asian bias in species diversity of temperate plants. Nature 407:180–182. doi:10.1038/35025052
Qian CJ, Yin HX, Shi Y, Zhao JC, Yin CL, Luo WY, Dong ZB, Chen GX, Yan X, Wang XR, Ma XF (2016) Population dynamics of Agriophyllum squarrosum, a pioneer annual plant endemic to mobile sand dunes, in response to global climate change. Sci Rep 6:1–12. doi:10.1038/srep26613
Qiu YX, Fu CX, Comes HP (2011) Plant molecular phylogeography in China and adjacent regions: tracing the genetic imprints of Quaternary climate and environmental change in the world’s most diverse temperate flora. Molec Phylogenet Evol 59:225–244. doi:10.1016/j.ympev.2011.01.012
Rajora OP, Dancik BP (1992) Chloroplast DNA inheritance in Populus. Theor Appl Genet 84:280–285. doi:10.1007/BF00229483
Rebering CA, Schneeweiss GM, Bardy KE, Schonswetter P, Villasenor JL, Overnayer R, Stuessy TF, Weiss-Schneeweiss H (2010) Multiple Pleistocene refugia and Holocene range expansion of an abundant southwestern American desert plant species (Melampodium leucanthum, Asteraceae). Molec Ecol 19:3421–3443. doi:10.1111/j.1365-294X.2010.04754.x
Rhodes TE, Gasse F, Ruifen L, Fontes JC, Keqin W, Bertrand P, Gibert E, Mélières F, Tucholka P, Wang ZX, Cheng ZY (1996) A Late Pleistocene-Holocene lacustrine record from Lake Manas, Zunggar (northern Xinjiang, western China). Palaeogeogr Palaeoclimatol 120:105–121. doi:10.1016/0031-0182(95)00037-2
Richardson JE, Pennington RT, Pennington TD, Hollingsworth PM (2001) Rapid diversification of a species-rich genus of neotropical rain forest trees. Science 293:2242–2245. doi:10.1126/science.1061421
Riddle BR, Hafner DJ (2006) A step-wise approach to integrating phylogeographic and phylogenetic biogeographic perspectives on the history of a core North American warm deserts biota. J Arid Environm 66:435–461. doi:10.1016/j.jaridenv.2006.01.014
Riddle BR, Hafner DJ, Alexander LF, Jaeger JR (2000) Cryptic vicariance in the historical assembly of a Baja California Peninsular Desert biota. Proc Natl Acad Sci USA 97:14438–14443. doi:10.1073/pnas.250413397
Rogers AR, Harpending H (1992) Population growth makes waves in the distribution of pairwise genetic differences. Molec Biol Evol 9:552–569
Rundle HD, Nosil P (2005) Ecological speciation. Ecol Lett 8:336–352. doi:10.1111/j.1461-0248.2004.00715
Schluter D (2000) The ecology of adaptive radiation. Oxford Universty Press, Oxford
Schneider S, Excoffier L (1999) Estimation of past demographic parameters from the distribution of pairwise differences when the mutation rates vary among sites: application to human mitochondrial DNA. Genetics 152:1079–1089
Schonswetter P, Popp M, Brochmann C (2006) Rare arctic-alpine plants of the European alps have different immigration histories: the snow bed species Minuartia biflora and Ranunculus pygmaeus. Molec Ecol 15:709–720. doi:10.1111/j.1365-294X.2006.02821.x
Slatkin M, Hudson RR (1991) Pairwise comparisons of mitochondrial DNA sequences in stable and exponentially growing populations. Genetics 129:555–562
Small RL, Ryburn JA, Cronn RC, Seelanan T, Wendel JF (1998) The tortoise and the hare: choosing between noncoding plastome and nuclear Adh sequences for phylogeny reconstruction in a recently diverged plant group. Amer J Bot 85:1301–1315. doi:10.2307/2446640
Sosa V, Ruiz-Sanchez E, Rodriguez-Gomez FC (2009) Hidden phylogeographic complexity in the Sierra Madre Oriental: the case of the Mexican tulip poppy Hunnemannia fumariifolia (Papaveraceae). J Biogeogr 36:18–27. doi:10.1111/j.1365-2699.2008.01957.x
Stewart JR, Lister AM, Barnes I, Dalen L (2010) Refugia revisited: individualistic responses of species in space and time. Proc Biol Sci 277:661–671. doi:10.1098/rspb.2009.1272
Su ZH, Zhang ML, Cohen JI (2012) Phylogeographic and demographic effects of Quaternary climate oscillations in Hexinia polydichotoma (Asteraceae) in Tarim Basin and adjacent areas. Pl Syst Evol 298:1767–1776. doi:10.1007/s00606-012-0677-6
Sun H, Li ZM (2003) Qinghai-Tibet Plateau uplift and its impact on tethhys flora. Advances Earth Sci 18:852–862. doi:10.3321/j.issn:1001-8166.2003.06.004
Sun JM, Liu TS (2006) The age of the Taklimakan Desert. Science 312:1621. doi:10.1126/science.1124616
Sun JM, Ding ZL, Liu TS (1998) Desert distributions during the glacial maximum and climatic optimum: example of China. Episodes 21:28–31. doi:10.1130/G25338A.1
Sun JM, Zhang ZQ, Zhang LY (2009) New evidence on the age of the Taklimakan Desert. Geology 37:159–162. doi:10.1130/G25338A.1
Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123:585–595
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular Evolutionary Genetics Analysis (GEGA) software version 4.0. Molec Biol Evol 24:1596–1599. doi:10.1093/molbev/msm092
Thompson JD, Gibson TJ, Plewinak F, Jeanmougin F, Higgins DG (1997) The Clustal-X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl Acids Res 25:4876–4882. doi:10.1093/nar/25.24.4876
Tian B, Liu RR, Wang LY, Qiu Q, Chen KM, Liu JQ (2009) Phylogeographic analyses suggest that a deciduous species (Ostryopsis davidiana Decne., Betulaceae) survived in northern China during the last glacial maximum. J Biogeogr 36:2148–2155. doi:10.1111/j.1365-2699.2009.02157.x
Wan DS, Feng JJ, Jiang DC, Mao KS, Duan YW, Miehe G, Opgenoorth L (2016) The Quaternary evolutionary history, potential distribution dynamics, and conservation implications for a Qinghai-Tibet Plateau endemic herbaceous perennial, Anisodus tanguticus (Solanaceae). Ecol Evol 6:1977–1995. doi:10.1002/ece3.2019
Wang FT, Tang T, Chen SC, Xu JM, Liang SY, Tsi ZH, Lang KY, Mao ZM, Shue LZ (1980) Liliaceae. Flora of China (14). Science Press, Beijing, pp 224–226
Wang LY, Abbott RJ, Zheng W, Chen P, Wang YJ, Liu JQ (2009) History and evolution of alpine plants endemic to the Qinghai-Tibetan Plateau: Aconitum gymnandrum (Ranunculaceae). Molec Ecol 18:709–721. doi:10.1111/j.1365-294X.2008.04055.x
Wang Q, Yu QS, Liu JQ (2011) Are nuclear loci ideal for barcoding plants? A case study of genetic delimitation of two sister species using multiple loci and multiple intraspecific individuals. J Syst Evol 49:182–188. doi:10.1111/j.1759-6831.2011.00135.x
Wolfe KH, Li WH, Sharp PM (1987) Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast and nuclear DNAs. Proc Natl Acad Sci USA 84:9054–9058
Wright S (1978) Evolution and the genetics of populations 4. Variability within and among natural populations. University of Chicago Press, Chicago
Wu LL, Cui XK, Milne RI, Sun YS, Liu JQ (2010) Multiple autopolyploidizations and range expansion of Allium przewalskianum Regel. (Alliaceae) in the Qinghai-Tibetan Plateau. Molec Ecol 19:1691–1704. doi:10.1111/j.1365-294X.2010.04613.x
Xu C, Shen X, Xu Y (2007) An analysis of climate change in East Asia by using the IPCC AR4 simulations. Advances Clim Change Res 3:287–292. doi:10.3969/j.issn.1673-1719.2007.05.008
Yang XP, Zhu ZD, Jaekel D, Owen LA, Han JM (2002) Late Quaternary palaeoenvironment change and landscape evolution along the Keriya River, Xinjiang, China: the relationship between high mountain glaciation and landscape evolution in foreland desert regions. Quaternary Int 97–98:155–166. doi:10.1016/S1040-6182(02)00061-7
Yang D, Fang XM, Dong GR, Peng ZC, Li JJ (2006) Aeolian deposit evidence for formation and evolution of the Tengger Desert in the north of China since early Pleistocene. Mar Geol Quaternary Geol 26:93–100
Yu QS, Wang Q, Wang AL, Wu GL, Liu JQ (2010) Interspecific delimitation and phylogenetic origin of Pugionium (Brassicaceae). J Syst Evol 48:195–206. doi:10.1111/j.1759-6831.2010.00078.x
Yu QS, Wang Q, Wu GL, Ma YZ, He XY, Wang X, Xie PH, Hu LH, Liu JQ (2013) Genetic differentiation and delimitation of Pugionium dolabratum and Pugionium cornutum (Brassicaceae). Pl Syst Evol 299:1355–1365. doi:10.1007/s00606-013-0800-3
Zhang HX, Zhang ML (2012) Identifying a contact zone between two phylogeographic lineages of Clematis sibirica (Ranunculeae) in the Tianshan and Altai Mountains. J Syst Evol 50:295–304. doi:10.1111/j.1759-6831.2012.00198.x
Zhang Q, Chiang TY, George M, Liu JQ, Abbott RJ (2005) Phylogeography of the Qinghai-Tibetan Plateau endemic Juniperus przewalskii (Cupressaceae) inferred from chloroplast DNA sequence variation. Molec Ecol 14:3513–3524. doi:10.1111/j.1365-294X.2005.02677.x
Zhang W, Cui ZJ, Li YH, Wang ZL, Yu Y, He MY (2012) Quaternary glacier development and the relationship between the climate change and tectonic uplift in the Helan Mountain. Chin Sci Bull 57:4491–4504. doi:10.1007/s11434-012-5283-z
Zhang YH, Liu SZ, Yu QS, He FL, Zhang JH (2014) Geographical distribution and seed characteristics of Allium mongolicum in China. J Desert Res 34:391–395. doi:10.7522/j.issn.1000-694X.2013.00330
Acknowledgements
The authors thank Jianquan Liu (Lanzhou University) for his guidance and suggestions for this work, Huitao Liu (Cansas State University) for his English improvement in the manuscript, Li Feng (Northwest University) for his help in Ecological Niche modeling analysis and two anonymous reviewers for their constructive suggestions on revision. This research was supported by the National Natural Science Foundation of China (31360089, 31360098), the West Light Foundation of the Chinese Academy of Sciences and the Foundation for Innovation Research Groups of Gansu Province of China (145RJIA335).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict interest.
Additional information
Handling editor: Yunpeng Zhao.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Information on Electronic Supplementary Materials
Information on Electronic Supplementary Materials
Online resource 1. Sequences of the 14 chlorotypes (H1-H14) identified in Allium mongolicum, which were used for producing phylogeny tree. The last one is the sequence of Allium anisopodium (outgroup). These sequences are the combined alignments of the two chloroplast DNA fragments (accD-psaI and psbA-trnH).
Online resource 2. Localities used for predicting the past and present distribution of Allium mongolicum under maximum entropy modeling with MAXENT version 3.3.3k. 1-38, field sampling sites; 39-85, specimen records from the Chinese Virtual Herbarium.
Online resource 3. Phylogenetic relationships of fourteen chlorotypes resolved in A. mongolicum using A. anisopodium as outgroup.
Online resource 4. Percentage of variance among populations and genetic diversity indices estimated by SAMOVA.
Online resource 5. Estimates of relative contributions of the environmental variables to the MAXENT model.
Rights and permissions
About this article
Cite this article
Zhang, Y., Yu, Q., Zhang, Q. et al. Regional-scale differentiation and phylogeography of a desert plant Allium mongolicum (Liliaceae) inferred from chloroplast DNA sequence variation. Plant Syst Evol 303, 451–466 (2017). https://doi.org/10.1007/s00606-016-1383-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-016-1383-6