Abstract
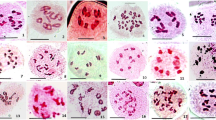
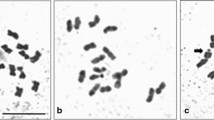
Campuloclinium macrocephalum DC. is a perennial herb widely distributed in the New World and introduced in South Africa, where it is commonly called “pompom weed”. This species is considered one of the most important weeds of Brazil and one of the problematic invasive plants of South Africa. The meiotic system can be studied to assess the ability of a weed to spread, but only few studies on C. macrocephalum have been realized. In this study, we examined the meiotic behavior and pollen fertility of 14 natural populations of C. macrocephalum from Argentina and Uruguay. Meiotic analysis revealed 2 triploid (2n = 3x = 30), 11 tetraploid (2n = 4x = 40) and 1 mixed population (2n = 2x = 20, 2n = 4x = 40). Both, triploid and tetraploid specimens showed a widely variable meiotic behavior with irregular chromosome pairing showing univalents, bivalents, trivalents (in triploids) and tetravalents (in tetraploids) at diacinesis of first meiotic division. Different abnormalities were observed, such as: laggard chromosomes, chromatin bridges, and out of plate chromosomes at metaphase I. During meiosis I (prophase), some cells showed the phenomenon of cytomixis or chromatin transfer between pollen mother cells. The meiotic indexes suggest that only four populations were normally fertile (over 90 % of fertile pollen), indicating meiotically stable plants. The remaining populations share variable pollen fertility, with triploids ranging from 46.64 to 54.83 % and tetraploids varying from 3.54 to 45.30 %. We suggest that polyploidy seems to be recurrent in C. macrocephalum, promoting partial sterility of pollen grains, generating large numbers of individuals by apomixis promoting invasion of crop fields. This study presents the meiotic behavior of this weed, these could be useful for future studies of biological control in areas with no natural enemies.

Similar content being viewed by others
References
Baker HG, Baker H, Stebbins G (1965) Characteristics and modes of origin of weeds. The genetics of colonizing species. 147–68
Baptista-Giacomelli FR, Pagliarini MS, de Almeida JL (2000) Meiotic behavior in several Brazilian oat cultivars (Avena sativa L.). Cytologia 65:371–378
Basavaiah D, Murthy TCS (1987) Cytomixis in pollen mother cells of Urochloa panicoides P. Beauv. (Poaceae). Cytologia 52:69–74
Bellucci MM, Roscini C, Mariani A (2003) Cytomixis in pollen mother cells of Medicago sativa L. J Hered 94(6):512–516
Bertasso Borges MS, Coleman JR (1998) Embryology and cytogenetics of Eupatorium pauciflorum and E. intermedium (Compositae). Genet Mol Biol 21:507–514
Bertasso Borges MS, Coleman JR (2005) Cytogenetics and embryology of Eupatorium laevigatum (Compositae). Genet Mol Biol 28:123–128
Bougourd SM, Jones RN (1997) B chromosomes a physiological. New Phytol 137:43–54
Burdon JJ, Groves R, Cullen JM (1981) The impact of biological control on the distribution and abundance of Chondrilla juncea in south-eastern Australia. J Appl Ecol:957–966
Cabrera AL (1974) Compositae. In: Burkart A. Fl Ilustr Entre Ríos 6, 106–540
Cabrero J, Camacho JPM (2009) Cromosomas parásitos. Investigación y Ciencia, 41
Camacho JPM, Parker J (1993) First B Chromosome Conference. Miraflores de la Sierra, Madrid
Camacho JPM, Sharbel TF, Beukeboom LW (2000) B-chromosome evolution. Philos Trans R Soc Lond B Biol Sci 355(1394):163–178
Chapman HM, Parh D, Oraguzie N (2000) Genetic structure and colonizing success of a clonal, weedy species, Pilosella officinarum (Asteraceae). Heredity 84(4):401–409
Coleman JR (1989) Embryology and cytogenetics of apomictic hexaploid Eupatorium odoratum L. (Compositae). Braz J Genet 12:803–817
Coleman JR, Coleman MA (1984) Apomixis in two triploid Brazilian species of Eupatorium: E. bupleurifolium and E. callilepis. Braz J Genet 7:549–567
Coleman JR, Coleman MA (1988) Embryology and cytogenetics of apomictic triploid Eupatorium squalidum DC. (Compositae). Braz J Genet 11:129–148
Costas-Lippmann M (1979) Embryogeny of Cortaderia selloana and C. jubata (Gramineae). Botanical gazette:393–397
de Nettancourt D, Grant WF (1964) Lacytogénétiquede Lotus (Leguminosae) III. Un cas de cytomixie dans un hybride interspécifi que. Cytologia 29:191–195
Dematteis M, Molero J, Ângulo MB, Rovira AM (2007) Chromosome studies on some Asteraceae from South America. Bot J Linn Soc 153:221–230
Drewitz JJ, DiTomaso JM (2004) Seed biology of jubatagrass (Cortaderia jubata). Weed Sci 52:525–530
Farco GE, Dematteis M (2011) Análisis mitótico de tres citotipos diferentes de Campuloclinium macrocephalum (Eupatorieae, Asteraceae). Bol Soc Argent 46(Suplemento):56
Farco GE, Sosa MM, Dematteis M, Fernández A (2012) Cytology and embryology of the pompom weed, Campuloclinium macrocephalum (Eupatorieae, Asteraceae). S Afr J Bot 78:21–29
Fornasari L (1996) Ecology of old world hawkweeds, Hieracium species (Asteraceae), in their homeland and consideration on their potential weediness. In: Proceedings of the IX International Symposium on Biological Control of Weeds. University of Cape Town, Stellenbosch, p 11-18
Freire SE (2008) Tribu Eupatorieae (Asteraceae). In: Zuloaga FO, Morrone O, Belgrano M (eds.) Catálogo de las Plantas Vasculares del Cono Sur de América del Sur: Argentina, Sur de Brasil (Paraná, Santa Catarina y Rio Grande do Sul), Chile, Paraguay y Uruguay. Monographs in Systematic Botany from the Missouri Botanical Garden 114, 1277–1302
Galiano NG, Hunziker JH (1987) Estudios cariológicos en Compositae IV Vernonieae y Eupatorieae. Darwiniana 28:1–8
Goodall J, Witkowski ETF, McConnachie AJ, Keen C (2012) Altered growth, population structure and realised niche of the weed Campuloclinium macrocephalum (Asteraceae) after exposure to the naturalised rust Puccinia eupatorii (Pucciniaceae). Biol Invasions 14(9):1947–1962
Halverson K, Heard SB, Nason JD, Stireman JO (2008) Origin, distribution, and local co-occurrence of polyploid cytotypes in Solidago altissima (Asteraceae). Am J Bot 95:50–58
Haroun SA (1995) Cytomixis in pollen mother cells of Polygonum tomentosum Schrank. Cytologia 60:257–260
Haroun SA, Al Shehri AM, Al Wadie HM (2004) Cytomixis in the microsporogénesis of Vicia faba L. (Fabaceae). Cytologia 69:7–11
Hodálová I, Grulich V, Horová L, Valachovič M, Marhold K (2007) Occurrence of tetraploid and octoploid cytotypes of Senecio jacobaea ssp. jacobaea (Asteraceae) in Pannonia and the Carpathians. Bot J Linn Soc 154:231–242
Holmgren I (1919) Zytologische studien uber die fortpflanzung bei den gattungen Erigeron und Eupatorium. Svenska Vetenskapsakademiens Årsbok 59:1–118
Husband BC, Schemske DW (2000) Ecological mechanisms of reproductive isolation between diploid and tetraploid Chamerion angustifolium. J Ecol 88:689–701
Jones RN (1995) B chromosomes in plants. New Phytologist 131:411–434
Kaur D, Singhal VK (2012) Phenomenon of cytomixis and intraspecific polyploidy (2x, 4x) in Spergularia diandra (Guss.) Heldr. & Sart. in the cold desert regions of Kinnaur district (Himachal Pradesh). Cytologia 77:163–171
Keeler KH, Davis GA (1999) Comparison of common cytotypes of Andropogon gerardii (Andropogoneae, Poaceae). Am J Bot 86:974–979
Khonglam A, Singh A (1980). Cytogenetic studies on the weed species of Eupatorium found in Meghalaya, India. Proceedings: Plant Sciences, 89(4), 237-241
Khush GS (1973) Cytogenetics of aneuploids. Academic, New York
Kumar G, Tripathi R (2008) Induced cytomictic variations through abiotic stresses in grasspea (Lathyrus sativus L.). Indian J Genet 68:58–64
Kumar P, Singhal VK, Kaur D, Kaur S (2010) Cytomixis and associated meiotic abnormalities affecting pollen fertility in Clematis orientalis. Biol Plantarum 54(1):181–184
Levin DA (1975) Minority cytotypes exclusion in local plant populations. Taxon 24:35–43
Li XF, Song ZQ, Feng DS, Wang GH (2009) Cytomixis in Thinopyrum intermedium, Thinopyrum ponticum and its hybrids with wheat. Cereal Res Commun 37:353–361
Malallah GA, Attia TA (2003) Cytomixis and its possible evolutionary role in a Kuwaiti populations of Diplotaxis harra (Brassicaceae). Bot J Linn Soc 143:169–175
Mandal A, Datta AK (2012) Inter-and intra-plant variations in cytomictic behavior of chromosomes in Corchorus fascicularis Lamk. (Tiliaceae). Cytologia 77(2):269
Mandal A, Datta AK, Gupta S, Paul R, Saha A, Ghosh BK, Iqbal M (2013) Cytomixis—a unique phenomenon in animal and plant. Protoplasma 250(5):985–996
Mantu DE, Sharma AK (1983) Cytomixis in pollen mother cells of an apomictic ornamental Ervatamia divaricata (Linn.) Alston. Cytologia 48:201–207
Marks GE (1954) An acetic-carmine glycerol jelly for use in pollen fertility counts. Stain Technol 29:277
McConnachie AJ, Retief E, Henderson L, McKay F (2011) The initiation of a biological control programme against pompom weed, Campuloclinium macrocephalum (Less.) DC. (Asteraceae), in South Africa. Afr Entomol 19(2):258–268
Miljajev EL (1967) Cytochimiceskoje I electron–mikroskopi ceskoje izucenje mikosporogeneza Citrus sinensis. Autoreferat Kandidaatskej dizertacie
Morisset P (1978) Cytomixis in the pollen mother cells of Ononis (Leguminosae). Can J Genet Cytol 20:383–388
Nirmala A, Rao PN (1996) Genesis of chromosome numerical mosaicism in higher plants. Nucleus 39:15–175
Noyes R (2007) Apomixis in the Asteraceae: diamonds in the rough. Functional Func Plant Sci Biotech 1(2):207–222
Pritchard T (1960) Race formation in weedy species with special reference to Euphorbia cyparissias L. and Hypericum perforatum L. In: Biology of Weeds, Symp Brit Ecol Soc, p 61–66
Richards AJ (1970) Eutriploid facultative agamospermy in Taraxacum. New Phytol 69(3):761–774
Rozenblum E, Maldonado S, Waisman CE (1988) Apomixis in Eupatorium tanacetifolium (Compositae). Am J Bot 75:311–322
Saggoo MIS, Gill A, Walia S (2011) Cytomixis during microsporogenesis in some populations of Croton bonplandianum of north India. Cytologia 76(1):67–72
Satina S, Blakeslee AF (1937) Chromosome behavior in triploids of Datura stramonium. I. The male gametophyte. Amer J Bot 24:518–527
Semyarkhina SYA, Kuptsou MS (1974) Cytomixis in various forms of sugarbeet. Vests I ANBSSE Ser Biyal 4:43–47
Singh RN (1992) Chromosomal abnormalities and fertility in induced autotetraploid Helianthus annuus in the C1 and C2 generations. Cytologia 57:277–281
Singhal VK, Kaur D (2011) Cytomixis induced meiotic irregularities and pollen malformation in Clematis graveolens Lindley from the cold deserts of Kinnaur district of Himachal Pradesh (India). Cytologia 76(3):319–327
Singhal VK, Kumar P (2008) Impact of cytomixis on meiosis, pollen viability and pollen size in wild populations of Himalayan poppy (Meconopsis aculeata Royle). J Biosciences 33:371–380
Singhal VK, Gill BS, Dhaliwal RS (2007) Status of chromosomal diversity in the hardwood tree species of Punjab state. J Cytol Genet 8:67–83
Singhal VK, Kaur S, Kumar P (2010) Aberrant male meiosis, pollen sterility and variable sized pollen grains in Clematis montana Buch.-Ham. ex DC. from Dalhousie hills, Himachal Pradesh. Cytologia 75:31–36
Sparvoli E (1960) Osservazioni cito-embriologiche in Eupatorium riparium. II. Megasporogenesi e sviluppo del gametofito femminile. Ann Bot 26:481–504
Srivastava P, Kumar G (2011) EMS-induced cytomictic variability in safflower (Carthamus tinctorius L.). Cytol Genet 45(4):240–244
Suda J (1998) Taxonomická problematika rodu Oxycoccus v České republice se zvláštním zřetelem kúzemí Šumavy. Zpr Čes Bot Společ 32:189–195
Suda J, Lysák MA (2001) A taxonomic study of the Vaccinium sect. Oxycoccus (Hill) W. D. J. Koch (Ericaceae) in the Czech Republic and adjacent territories. Folia Geobot 36:303–320
Takats ST (1959) Chromatin extrusion and DNA transfer during microsporogenesis. Chromosoma 10:430–453
Turner BL, Bacon J, Urbatsh L, Simpson B (1979) Chromosome numbers in South American compositae. Am J Bot 66:173–178
Van Dijk P, Bakx-Schotman T (1997) Chloroplast DNA phylogeography and cytotype geography in autopolyploid Plantago media. Mol Ecol 6:191–199
Weiling F (1965) Light and electron microscopical observation on cytomixis and its possible relation topotocytosis. Planta 67:182–212
Zhen GS, Li XF (2009) Cytomixis in pollen mother cells of Salvia miltiorrhiza. Caryologia 62(3):213–219
Acknowledgments
This work has been supported by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and the Secretarıa General de Ciencia y Tecnica of the Universidad Nacional del Nordeste, which are greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farco, G.E., Dematteis, M. Meiotic behavior and pollen fertility in triploid and tetraploid natural populations of Campuloclinium macrocephalum (Eupatorieae, Asteraceae). Plant Syst Evol 300, 1843–1852 (2014). https://doi.org/10.1007/s00606-014-1011-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-014-1011-2