Abstract
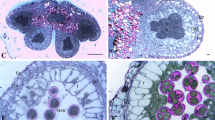
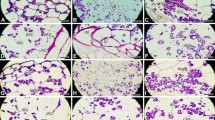
In this study, distribution of polysaccharides, lipids, and proteins in the developing anthers of Campsis radicans (L.) Seem. was examined from sporogenous cell stage to mature pollen, using cytochemical methods. To detect the distribution and dynamic changes of insoluble polysaccharides, lipid bodies, and proteins in the anthers through progressive developmental stages, semi-thin sections of anthers at different developmental stages were stained with periodic-acid-Schiff (PAS) reagent, Sudan black B, and Coomassie brilliant blue, respectively, and examined under light microscope. Ultrastructural observations with TEM were also carried out to determine the storage form of starch in the connective tissue, and storage form of lipids in the tapetal cells. In sporogenous cell stage, anther wall contains numerous insoluble polysaccharides. However, from the sporogenous cell stage to the vacuolated microspore stage, the amount of insoluble polysaccharides in the anther wall decreases gradually. At bicellular pollen stage, tapetum degenerates completely and polysaccharides are not seen in the anther wall. Lipid bodies are observed in the cytoplasm of both middle layer and tapetal cells at tetrad stage, whereas they disappear in the vacuolated microspore stage. Compared with polysaccharides, proteins are limited in the anther wall at early stages of development. During pollen development, polysaccharides, proteins, and lipid bodies are scarce in the cytoplasm of sporogenous cells, but their amount increases at premeiotic stage. From tetrad stage to bicellular pollen stage, microspore cytoplasm contains variable amount of insoluble polysaccharide grains, lipid and protein bodies. At bicellular pollen stage, plentiful amount of starch granules are stored in the cytoplasm of the pollen grains. Proteins and lipid bodies are also present in the cytoplasm.


















Similar content being viewed by others
References
Aybeke M (2012) Anther wall and pollen development in Ophrys mammosa L. (Orchidaceae). Plant Syst Evol 298:1015–1023
Baker HG, Baker I (1979) Starch in angiosperm pollen grains and) its evolutionary significance. Am J Bot 5:591–600
Bhandari NN (1984) The microsporangium. In: Johri BM (ed) The Embryology of Angiosperms. Berlin, Heidelberg, pp 53–157
Castro A, Clément C (2007) Sucrose and starch catabolism in the anther of Lilium during its development: a comparative study among the anther wall, locular fluid and microspore/pollen fractions. Planta 225:1573–1582
Chen BX, Zhao CH, Zhao YY (2012) Pollen morphology and ontogeny of Maianthemum bifolium (L.) F.W. Schmidt in China. Plant Syst Evol. doi:10.1007/s00606-012-0709-2
Clément C, Audran JC (1995) Anther wall layers control pollen sugar nutrition in Lilium. Protoplasma 187:172–181
Clément C, Chavant L, Burrus M, Audran JC (1994) Anther starch variations in Lilium during pollen development. Sex Plant Reprod 7:347–356
Datta R, Chamusco KC, Chourey PS (2002) Starch biosynthesis during pollen maturation is associated with altered patterns of gene expression in maize. Plant Physiol 130:1645–1656
Dorion S, Lalonde S, Saini HS (1996) Induction of male sterility in wheat by meiotic stage water deWcit is preceded by a decline in invertase activity and changes in carbohydrate metabolism in anthers. Plant Physiol 111:137–145
Ekici N, Dane F (2012) Some histochemical features of anther wall of Leucojum aestivum (Amaryllidaceae) during pollen development. Biologia 67(5):857–866
Galati BG, Zarlavsky G, Rosenfeldt S, Gotelli MM (2012) Pollen ontogeny in Magnolia liliflora. Desr Plant Syst Evol 298:527–534
Geitmann A, Hudák J, Vennigerholz F, Walles B (1995) Immunogold localization of pectin and callose in pollen grains and pollen tubes of Brugmansia suaveolens. Implications for the self-incompatibility reaction. J Plant Physiol 147:225–235
Glauert MA, Glauert RH (1958) Araldite as an embedding medium for electron microscopy. J Biophys Biochem Cytol 4:191
Goldberg RB, Beals TP, Sanders PM (1993) Anther development: basic principles and practical applications. Plant Cell 5:1217–1229
Gotelli M, Galati B, Medan D (2012) Pollen, tapetum, and orbicule development in Colletia paradoxa and Discaria americana (Rhamnaceae). Sci World J, vol 2012. doi:10.1100/2012/948469
Halbritter H (2000) Onwards. Campsis radicans. In: Buchner R, Weber M. PalDat—a palynological database: Descriptions,illustrations, identifi cation, and information retrieval. http://paldat.botanik.univie.ac.at
Hegde R, Bendigeri P, Gaonkar S, Needigi P, Katti R, Agadi S, Agadi B (2000) Significance of unusual features of the anthers of spathoglottis plicata bl. Cytologia 65:103–112
Jinliang H, Hanqing X, Qingya W, Qingyuan H (1994) Studies on the development and histochemistry of anther and pollen in Prunus mume Sieb. et Zucc. J Nanjing Agric Univ 17(4):7–12
Liu RS, Qiu YL, Wei DM, Liu HH, Zhu XY, Tian HQ, Teixeira da Silva JA (2011) Distribution of starch and neutral lipids in the developing anthers of Ipomoea cairica. Ann Bot Fennici 48:256–262
Liu JX, Wang M, Chen BX, Jin P, Li JY, Zeng K (2012) Microsporogenesis, microgametogenesis, and pollen morphology of Ambrosia artemisiifolia L. in China. Plant Syst Evol 298:43–50
Mascarenhas JP (1989) The male gametophyte of flowering plants. Plant Cell 1:657–664
Miki-Hiroshige H, Nakamura S (1981) The metabolic incorporation of label from myoinositol-2-3H by the growing young anther of Lilium longiflorum. Ada Soc Bot Pol 50:77–82
Mousavi A, Hiratsuka R, Takase H, Hiratsuka K, Hotta Y (1999) A novel glycine-rich protein is associated with starch grain accumulation during anther development. Plant Cell Physiol 40(4):406–416
Noher de Halac I, Fama G, Cismondi IA (1992) Changes in lipids and polysaccharides during pollen ontogeny in Oenothera anthers. Sex Plant Reprod 5:110–116
Pacini E (1990) Tapetum and microspore function. In: Blackmore S, Knox RB (eds) Microspores: evolution and ontogeny. Academic press, London, pp 213–237
Pacini E (1996) Types and meaning of pollen carbohydrate reserves. Sex Plant Reprod 9:362–366
Pacini E, Franchi GG, Hesse M (1985) The tapetum: its form, function, and possible phylogeny in Embryophyta. Plant Syst Evol 149:155–185
Panchaksharappa MG, Rudramuniyappa CK (1974) Localization of nucleic acids and insoluble polisaccharides in the anther of Zea mays L. A histochemical study. Cytologia 39:153–160
Reznickova SA (1983) Metabolism of reserve substances in the developing anther. In: Erdelska O (ed) Fertilization and Embryogenesis in Ovulated Plants. Bratislava, Veda, pp 57–62
Reznickova SA, Willemse MTM (1980) The formation of pollen in the anther of Lilium. II. The function of the surrounding tissues in the formation of pollen and pollen wall. Acta Bot Need 29:14–156
Reznikova SA, Willemse MTM (1982) Electron-microscopic and histochemical investigation of tissues of the developing lily anther in connection with metabolism of reserve nutrient substances. All Union Scientific Reserve Institute of Essential Oil Crops. Simferopol, Landbouwhogeschool, Wageningen, The Netherlands. Plenum Press, New York, pp 856–863
Saini HS (1997) Effects of water stress on male gametophyte development in plants. Sex Plant Reprod 10:67–73
Saini HS, Lalonde S (1998) Injuries to reproductive development under water stress, and their consequences for crop productivity. J Crop Prod 1:223–248
Schrauwen JAM, Mettenmeyer T, Croes AF, Wullems GJ (1996) Tapetum-specific genes: what role do they play in male gametophyte development. Acta Bot Neerl 45:1–15
Tütüncü Konyar S, Dane F (2013a) Cytochemistry of pollen development in Campsis radicans (L.) Seem. (Bignoniaceae). Plant Syst Evol 299:87–95
Tütüncü Konyar S, Dane F (2013b) Anther ontogeny in Campsis radicans (L.) Seem. (Bignoniaceae) Plant Syst Evol. doi:10.1007/s00606-012-0743-0
Tütüncü S, Dane F, Tütüncü S (2007) Examination of pollen morphology of some exotic trees and shrubs found in the parks and the gardens of Edirne (European Turkey) I. J Appl Biol Sci I(2):45–55
Vardar F, Ünal M (2011) Cytochemical and ultrastructural observations of anthers and pollen grains in Lathyrus undulatus Boiss. Acta Botanica Croatica 70:53–64
Vardar F, İsmailoğlu I, Ünal M (2012) Anther development and cytochemistry in Asphodelus aestivus (Asphodelaceae). Turkish J Bot. doi:10.3906/bot-1202-20
Xie CT, Yang YH, Qiu YL, Zhu XY, Tian HQ (2005) Cytochemical investigation of genic male-sterility in Chinese cabbage. Sex Plant Reprod 18:75–80
Xu Q, Wang XQ, Tian HO (2006) The features of distribution of starch and lipid in the developing anthers of Lycium barbarum L. J Mol Cell Biol 39:103–110
Yeung EC, Oinam GS, Yeung SS, Harry I (2011) Anther, pollen and tapetum development in safflower, Carthamus tinctorius L. Sex Plant Reprod 24:307–317
Acknowledgments
We would like to thank the Scientific Research Fund of Trakya University, which financially supported this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tütüncü Konyar, S., Dane, F. & Tütüncü, S. Distribution of insoluble polysaccharides, neutral lipids, and proteins in the developing anthers of Campsis radicans (L.) Seem. (Bignoniaceae). Plant Syst Evol 299, 743–760 (2013). https://doi.org/10.1007/s00606-013-0758-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-013-0758-1