Abstract
The genetic relationships among the sedges Carex aquatilis, C. paleacea, and C. recta, and C. recta and other hybrids (C. aquatilis × recta and C. paleacea × recta) were investigated. Microsatellite-based, dominant inter simple sequence repeat (ISSR) fingerprinting revealed no taxon-specific variation among five investigated Carex species or hybrids, while in codominant microsatellite genotyping, 5 out of 16 alleles were taxon specific. Despite significant genetic differentiation, our results demonstrate that there is also gene flow between taxa. Although principal component analysis (PCA) showed some structuring among groups, there was considerable overlapping. The analysis of genetic differentiation assessed for the expected five groups using the program Structure did not provide conclusive results for the number of main groups. However, it is visible that C. aquatilis and C. paleacea are genetically distinct, while the other studied taxa show intermediate genetic features. Both the microsatellite analysis and sequencing of the internal transcribed spacer (ITS) region supported the view that the parental species of C. recta are C. aquatilis and C. paleacea, although with unequal contribution. C. paleacea × recta is likely to possess additional genetic material apart from that originating from C. aquatilis and C. paleacea. Our study shows that ISSR markers failed to be sufficiently informative, but the combination of sequencing (ITS) and the use of microsatellite genotyping proved their applicability in resolving fine-scale taxonomic questions.



Similar content being viewed by others
References
Ball PW, Reznicek AA (2002) Carex. In: Flora of North America Editorial Committee (ed) Flora of North America. Oxford University Press, New York, pp 254–273
Barker MS, Hauk WD (2003) An evaluation of Sceptridium dissectum (Ophioglossaceae) with ISSR markers: implications for Sceptridium systematics. Am Fern J 93:1–19
Basha SD, Sujatha M (2009) Genetic analysis of Jatropha species and interspecific hybrids of Jatropha curcas using nuclear and organelle specific markers. Euphytica 168:197–214
Cayouette J, Catling PM (1992) Hybridization in the genus Carex with special reference to North America. Bot Rew 58:351–438
Cayouette J, Morisset P (1985) Chromosome studies on natural hybrids between maritime species of Carex (sections Phacocystis and Cryptocarpae) in northeastern North America, and their taxonomic implications. Can J Bot 63:1957–1982
Cayouette J, Morisset P (1986a) Chromosome studies on Carex salina complex (Cyperaceae, sections Cryptocarpae) in northeastern North America. Cytologia 51:817–856
Cayouette J, Morisset P (1986b) Chromosome studies on Carex paleacea Wahl., C. nigra (L.) Reichard, and C. aquatilis Wahl. in northeastern North America. Cytologia 51:857–883
Choler P, Erschbamer B, Tribsch A, Gielly L, Taberlet P (2004) Genetic introgression as a potential to widen a species’ niche: insights from alpine Carex curvula. Proc Natl Acad Sci USA 101:171–176
Dean M, Ashton PA (2008) Leaf surfaces as a taxonomic tool: the case of Carex section Phacocystis (Cyperaceae) in the British Isles. Plant Syst Evol 273:97–105
Dean M, Ashton PA, Hutcheon K, Jermy AC, Cayotte J (2008) Description, ecology and establishment of Carex salina Wahlenb. (Saltmarsh Sedge)—a new British species. Watsonia 27:51–57
Egorova TV (1999) The sedges (Carex L.) of Russia and adjacent states (within the limits of the former USSR). St. Petersburg State Chemical-Pharmaceutical Academy, St. Petersburg & Missouri Botanical Garden Press, St. Louis
Elven R, Murray DF, Razzhivin V, Yurtsev BA (2005) Checklist of the panarctic flora (PAF): vascular plants. University of Oslo, Oslo
Ericson L, Wallentinus H-G (1979) Seashore vegetation around the Gulf of Bothnia. Guide for the International Society of Vegetation Science, July–August 1977. Wahlenbergia 5:1–142
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 13:2611–2620
Excoffier L, Laval G, Schneider S (2005) Arlequin Ver. 3.0: An integrated software package for population genetics data analysis. Computational and Molecular Population Genetics Lab, University of Berne, Switzerland
Falush D, Stephens M, Pritchard JK (2003) Inference of population structure: extensions to linked loci and correlated allele frequencies. Genetics 164:1567–1587
Faulkner JS (1972) Chromosome studies on Carex section Acutae in north-west Europe. Bot J Linn Soc 65:271–301
Faulkner JS (1973) Experimental hybridization of north-west European species in Carex section Acutae (Cyperaceae). Bot J Linn Soc 67:233–253
Fox CW, Wolf JB (2006) Evolutionary genetics: concepts and case studies. Oxford University Press, New York
Goldman JJ (2008) The use of ISSR markers to identify Texas bluegrass interspecific hybrids. Plant Breed 127:644–646
Hendrichs M, Michalski S, Begerow D, Oberwinkler F, Hellwig FH (2004a) Phylogenetic relationships in Carex, subgenus Vignea (Cyperaceae), based on ITS sequences. Plant Syst Evol 246:109–125
Hendrichs M, Oberwinkler F, Begerow D, Bauer R (2004b) Carex, subgenus Carex (Cyperaceae)—a phylogenetic approach using ITS sequences. Plant Syst Evol 246:89–107
Hipp AL, Kettenring KM, Feldheim KA, Weber JA (2009) Isolation of 11 polymorphic tri- and tetranucleotide microsatellite loci in a North American sedge (Carex scoparia: Cyperaceae) and cross-species amplification in three additional Carex species. Mol Ecol Res 9:625–627
Johansson MM, Kahma KK, Boman H, Launiainen J (2004) Scenarios for sea level on the Finnish coast. Boreal Envir Res 9:153–166
King MG, Roalson EH (2009) Isolation and characterization of 11 microsatellite loci from Carex macrocephala (Cyperaceae). Conserv Genet. doi:10.1007/s10592-008-9558-5
Korpelainen H, Virtanen V, Kostamo K, Karttunen H (2008) Molecular evidence shows that the moss Rhytidiadelphus subpinnatus (Hylocomiaceae) is clearly distinct from R. squarrosus. Mol Phylog Evol 48:372–376
Kress WJ, Erickson DL (2007) A two-locus global DNA barcode for land plants: the coding rbcL gene complements the non-coding trnH-psbA spacer region. PLoS ONE 6:e508
Lexer C, Widmer A (2008) The genic view of plant speciation: recent progress and emerging questions. Philos Trans R Soc B Biol Sci 363:3023–3036
McClintock KA, Waterway MJ (1994) Genetic differentiation between Carex lasiocarpa and C. pellita (Cyperaceae) in North America. Am J Bot 81:224–231
Nakamatte E, Arnstein Lye K (2007) AFLP-based differentiation in north Atlantic species of Carex sect. Phacocystis. Nordic J Bot 25:318–328
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Ohbayashi K, Hodoki Y, Nakayama S, Shimada M, Kunii H (2008) Development of new microsatellite markers from a salt-marsh sedge Carex rugulosa by compound simple sequence repeat-polymerase chain reaction. Mol Ecol Res 8:1497–1499
Ohsako T, Yamane K (2007) Isolation and characterization of polymorphic microsatellite loci in Asiatic sand sedge, Carex kobomugi Ohwi (Cyperaceae). Mol Ecol Notes 7:1023–1025
Otto SP, Whitton J (2000) Polyploid incidence and evolution. Ann Rev Genet 34:401–437
Paun O, Forest F, Fay MF, Chase MW (2009) Hybrid speciation in angiosperms: parental divergence drives ploidy. New Phytol 182:507–518
Rautiainen P, Aikio S, Hyvärinen M (2007) Growth and disturbance in the spatial dynamics of an endangered seashore grass Arctophila fulva var. pendulina. Ecol Model 207:145–154
Reznicek AA (1990) Evolution in sedges (Carex, Cyperaceae). Can J Bot 68:1409–1432
Rieseberg LH, Willis JH (2007) Plant speciation. Science 317:910–914
Roalson EH, Friar EA (2004) Phylogenetic relationships and biogeographic patterns in North American members of Carex section Acrocystis (Cyperaceae) using nrDNA ITS and ETS sequence data. Plant Syst Evol 243:175–187
SAS Institute Inc (2008) SAS/STAT 9.2 User’s guide. SAS Institute, Cary
Saxén U (1938) Die Varietäten von Carex salina Wg ssp. cuspidata Wg nebst ihren Hybriden an den Kusten des Bottnischen Busens Finnland. Acta Bot Fennica 22:130
Schönswetter P, Elven R, Brochmann C (2008) Trans-Atlantic dispersal and large-scale lack of genetic strcuture in the circumpolar, arctic-alpine sedge Carex bigelowii S. L. (Cyperaceae). Am J Bot 95:1006–1014
Slatkin M (1995) A measure of population subdivision based on microsatellite allele frequencies. Genetics 139:457–462
Smith TW, Waterway MJ (2008) Evaluating species limits and hybridization in the Carex complanata complex using morphology, amplified fragment length polymorphisms, and restriction fragment analysis. Botany 8:809–826
Soltis DE, Soltis PS (1999) Polyploidy: recurrent formation and genome evolution. Trends Ecol Evol 14:348–352
Standley LA (1990) Allozyme evidence for the hybrid origin of the maritime species of Carex salina and Carex recta (Cyperaceae) in eastern North America. Syst Bot 15:182–191
Strasburg JL, Rieseberg LH (2008) Molecular demographic history of the annual sunflowers Helianthus annuus and H. petiolaris—large effective population sizes and rates of long-term gene flow. Evolution 62:1936–1950
Sylvén N (1963) Det Skandinaviska flora-områdets Carices distidmaticae. Opera Botanica 8:1–161
Thompson JD, Higgins DG, Gibson TJ (1994) Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl Acids Res 22:4673–4680
Väre H (2007) Typification of names published by the Finnish botanist Fredrik Nylander. Ann Bot Fennici 44:465–480
Volkova PA, Shipunov AB, Elven R, Brochmann C (2008) The seashore sedges of the Russian Kola Pensinsula: How many species? Flora 203:523–533
Waterway MJ (1994) Evidence for the hybrid origin of Carex knieskernii with comments on hybridization in the genus Carex (Cyperaceae). Can J Bot 72:860–871
Waterway MJ, Starr JR (2007) Phylogenetic relationships in tribe Cariceae (Cyperaceae) based on nested analyses of four molecular data sets. Aliso 23:165–192
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand D, Sninsky J, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, San Diego, pp 315–322
Whitlock MC, McCauley DE (1999) Indirect measures of gene flow and migration: F ST ≠ 1/(4Nm + 1). Heredity 82:117–125
Acknowledgments
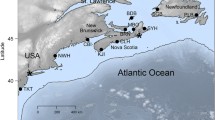
The project was funded by the Societas pro Fauna et Flora Fennica and the University of Helsinki. We wish to thank Tauno Ulvinen and Erkki Vilpa for help in collecting the material, Katja Schäfer for assistance in the laboratory, and Leena Helynranta for preparing the map of collection sites.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Korpelainen, H., Virtanen, V., Kostamo, K. et al. Hybridization and introgression in Carex aquatilis and C. paleacea . Plant Syst Evol 287, 141–151 (2010). https://doi.org/10.1007/s00606-010-0307-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-010-0307-0