Abstract
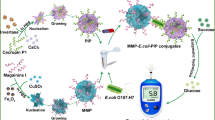
The potential of enzyme-encapsulated metal–organic framework (MOF) as an antibody label for the construction of enzyme-linked immunosorbent assay (ELISA) is demonstrated. Zeolitic imidazolate framework-90 (ZIF-90) was employed as a MOF model to load urease and pig immunoglobulin G (IgG) antibody. This leads to the production of U@ZIF-90/IgG composite, in which urease was encapsulated in ZIF-90 to form U@ZIF-90 for amplifying the detection signal, while IgG was anchored on the surface of U@ZIF-90 for specifically recognizing Staphylococcus aureus (S. aureus). Benefiting from the unique porous structure of ZIF-90, the U@ZIF-90 not only allows urease to be encapsulated with an ultrahigh loading efficiency, but also shields the loaded urease against harsh environments. The U@ZIF-90 shows a threefold higher catalytic activity than free urease due to the confinement effect. These findings lead to an ELISA with greatly enhanced sensitivity for S. aureus detection. By using a portable pH meter as a readout, the ELISA has a linear response that covers 10 to 109 CFU/mL S. aureus with a detection limit of 1.96 CFU/mL and exhibits high selectivity over other bacteria. The successful determination of S. aureus in milk samples demonstrates the applicability of the ELISA in a complex biological matrix.
Graphical abstract






Similar content being viewed by others
References
Hermann CA, Duerkop A, Baeumner AJ (2019) Food safety analysis enabled through biological and synthetic materials: a critical review of current trends. Anal Chem 91:569–587
Zhu H, Sikora U, Ozcan A (2012) Quantum dot enabled detection of Escherichia coli using a cell-phone. Analyst 137:2541–2544
Krishna VD, Wu K, Su D, Cheeran MCJ, Wang J-P, Perez A (2018) Nanotechnology: review of concepts and potential application of sensing platforms in food safety. Food Microbiol 75:47–54
Chen J, Andler SM, Goddard JM, Nugen SR, Rotello VM (2017) Integrating recognition elements with nanomaterials for bacteria sensing. Chem Soc Rev 46:1272–1283
Torun Ö, Hakkı Boyacı İ, Temür E, Tamer U (2012) Comparison of sensing strategies in SPR biosensor for rapid and sensitive enumeration of bacteria. Biosens Bioelectron 37:53–60
Su Y-l, Li J-r, Jiang L, Cao J (2005) Biosensor signal amplification of vesicles functionalized with glycolipid for colorimetric detection of Escherichia coli. J Colloid Interface Sci 284:114–119
Silbert L, Shlush IB, Israel E, Porgador A, Kolusheva S, Jelinek R (2006) Rapid chromatic detection of bacteria by use of a new biomimetic polymer sensor. Appl Environ Microb 72:7339–7344
Yoo SM, Kim D-K, Lee SY (2015) Aptamer-functionalized localized surface plasmon resonance sensor for the multiplexed detection of different bacterial species. Talanta 132:112–117
Duan YF, Ning Y, Song Y, Deng L (2014) Fluorescent aptasensor for the determination of Salmonella typhimurium based on a graphene oxide platform. Microchim Acta 181:647–653
Duan N, Wu S, Zhu C, Ma X, Wang Z, Yu Y, Jiang Y (2012) Dual-color upconversion fluorescence and aptamer-functionalized magnetic nanoparticles-based bioassay for the simultaneous detection of Salmonella Typhimurium and Staphylococcus aureus. Anal Chim Acta 723:1–6
Ouyang Q, Wang L, Ahmad W, Yang Y, Chen Q (2021) Upconversion nanoprobes based on a horseradish peroxidase-regulated dual-mode strategy for the ultrasensitive detection of Staphylococcus aureus in meat. J Agric Food Chem 69:9947–9956
Ye H, Yang K, Tao J, Liu Y, Zhang Q, Habibi S, Nie Z, Xia X (2017) An enzyme-free signal amplification technique for ultrasensitive colorimetric assay of disease biomarkers. ACS Nano 11:2052–2059
Jiang Y, Su Z, Zhang J, Cai M, Wu L (2018) A novel electrochemical immunoassay for carcinoembryonic antigen based on glucose oxidase-encapsulated nanogold hollow spheres with a pH meter readout. Analyst 143:5271–5277
Lin C, Guo Y, Zhao M, Sun M, Luo F, Guo L, Qiu B, Lin Z, Chen G (2017) Highly sensitive colorimetric immunosensor for influenza virus H5N1 based on enzyme-encapsulated liposome. Anal Chim Acta 963:112–118
Piao Y, Lee D, Kim J, Kim J, Hyeon T, Kim H-S (2009) High performance immunoassay using immobilized enzyme in nanoporous carbon. Analyst 134:926–932
Chen Y, Li P, Modica JA, Drout RJ, Farha OK (2018) Acid-resistant mesoporous metal-organic framework toward oral insulin delivery: protein encapsulation, protection, and release. J Am Chem Soc 140:5678–5681
Wang H-S (2017) Metal–organic frameworks for biosensing and bioimaging applications. Coord Chem Rev 349:139–155
Simon-Yarza T, Mielcarek A, Couvreur P, Serre C (2018) Nanoparticles of metal-organic frameworks: on the road to in vivo efficacy in biomedicine. Adv Mater 30:1707365
Li J-R, Sculley J, Zhou H-C (2012) Metal-organic frameworks for separations. Chem Rev 112:869–932
Wuttke S, Lismont M, Escudero A, Rungtaweevoranit B, Parak WJ (2017) Positioning metal-organic framework nanoparticles within the context of drug delivery - a comparison with mesoporous silica nanoparticles and dendrimers. Biomaterials 123:172–183
Gkaniatsou E, Sicard C, Ricoux R, Mahy J-P, Steunou N, Serre C (2017) Metal-organic frameworks: a novel host platform for enzymatic catalysis and detection. Mater Horiz 4:55–63
Huang S, Kou X, Shen J, Chen G, Ouyang G (2020) Armoring the enzymes with metal-organic frameworks. Angew Chem Int Ed 59:8786–8798
Lian X, Fang Y, Joseph E, Wang Q, Li J, Banerjee S, Lollar C, Wang X, Zhou H-C (2017) Enzyme-MOF (metal-organic framework) composites. Chem Soc Rev 46:3386–3401
Li P, Moon S-Y, Guelta MA, Harvey SP, Hupp JT, Farha OK (2016) Encapsulation of a nerve agent detoxifying enzyme by a mesoporous zirconium metal-organic framework engenders thermal and long-term stability. J Am Chem Soc 138:8052–8055
Li Z, Zhang Y, Su Y, Ouyang P, Ge J, Liu Z (2014) Spatial co-localization of multi-enzymes by inorganic nanocrystal-protein complexes. Chem Commun 50:12465–12468
Liao F-S, Lo W-S, Hsu Y-S, Wu C-C, Wang S-C, Shieh F-K, Morabito JV, Chou L-Y, Wu KCW, Tsung C-K (2017) Shielding against unfolding by embedding enzymes in metal-organic frameworks via a de novo approach. J Am Chem Soc 139:6530–6533
Weng Y, Song Z, Chen C-H, Tan H (2021) Hybrid hydrogel reactor with metal-organic framework for biomimetic cascade catalysis. Chem Eng J 425:131482
Cheng X, Zheng Z, Zhou X, Kuang Q (2020) Metal-organic framework as a compartmentalized integrated nanozyme reactor to enable high-performance cascade reactions for glucose detection. ACS Sustain Chem Eng 8:17783–17790
Weng Y, Zhu Q, Huang Z-Z, Tan H (2020) Time-resolved fluorescence detection of superoxide anions based on an enzyme-integrated lanthanide coordination polymer composite. ACS Appl Mater Interfaces 12:30882–30889
Liu G, Wang L, Zhu F, Liu Q, Feng Y, Zhao X, Chen M, Chen X (2022) Facile construction of a reusable multi-enzyme cascade bioreactor for effective fluorescence discrimination and quantitation of amino acid enantiomers. Chem Eng J 428:131975
Nishiyabu R, Hashimoto N, Cho T, Watanabe K, Yasunaga T, Endo A, Kaneko K, Niidome T, Murata M, Adachi C, Katayama Y, Hashizume M, Kimizuka N (2009) Nanoparticles of adaptive supramolecular networks self-assembled from nucleotides and lanthanide ions. J Am Chem Soc 131:2151–2158
Chang F-P, Hung Y, Chang J-H, Lin C-H, Mou C-Y (2014) Enzyme encapsulated hollow silica nanospheres for intracellular biocatalysis. ACS Appl Mater Interfaces 6:6883–6890
Chen W-H, Vázquez-González M, Zoabi A, Abu-Reziq R, Willner I (2018) Biocatalytic cascades driven by enzymes encapsulated in metal–organic framework nanoparticles. Nat Catal 1:689–695
Gao J, Wang C, Wang J, Tan H (2019) Cascade amplified time-resolved fluorescent assay driven by enzyme-integrated catalytic compartment as an artificial multi-enzyme complex. Chem Eur J 25:9629–9633
Chen S-Y, Lo W-S, Huang Y-D, Si X, Liao F-S, Lin S-W, Williams BP, Sun T-Q, Lin H-W, An Y, Sun T, Ma Y, Yang H-C, Chou L-Y, Shieh F-K, Tsung C-K (2020) Probing interactions between metal-organic frameworks and freestanding enzymes in a hollow structure. Nano Lett 20:6630–6635
Liang K, Ricco R, Doherty CM, Styles MJ, Bell S, Kirby N, Mudie S, Haylock D, Hill AJ, Doonan CJ, Falcaro P (2015) Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat Commun 6:7240
Zhang F-M, Dong H, Zhang X, Sun X-J, Liu M, Yang D-D, Liu X, Wei J-Z (2017) Postsynthetic modification of ZIF-90 for potential targeted codelivery of two anticancer drugs. ACS Appl Mater Interfaces 9:27332–27337
Zhang J, Lan T, Lu Y (2020) Translating in vitro diagnostics from centralized laboratories to point-of-care locations using commercially-available handheld meters. TrAC-Trends Anal Chem 124:115782
Kong W, Xiong J, Yue H, Fu Z (2015) Sandwich fluorimetric method for specific detection of Staphylococcus aureus based on antibiotic-affinity strategy. Anal Chem 87:9864–9868
Cheng D, Yu M, Fu F, Han W, Li G, Xie J, Song Y, Swihart MT, Song E (2016) Dual recognition strategy for specific and sensitive detection of bacteria using aptamer-coated magnetic beads and antibiotic-capped gold nanoclusters. Anal Chem 88:820–825
Wang Y, Liu X, Wu L, Ding L, Effah CY, Wu Y, Xiong Y, He L (2022) Construction and bioapplications of aptamer-based dual recognition strategy. Biosens Bioelectron 195:113661
Wang Z, Chen Z, Gao N, Ren J, Qu X (2015) Transmutation of personal glucose meters into portable and highly sensitive microbial pathogen detection platform. Small 11:4970–4975
Funding
This work was supported by the National Natural Science Foundation of China (22064011), the Scientific and Technological Innovation Talent Plan of Hunan Province (2021RC1015), and the Natural Science Foundation of Jiangxi Province (20202ACB205003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, S., Xie, H., Xie, F. et al. Immunoassay based on urease-encapsulated metal–organic framework for sensitive detection of foodborne pathogen with pH meter as a readout. Microchim Acta 189, 358 (2022). https://doi.org/10.1007/s00604-022-05462-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05462-8