Abstract
A sandwich immunoassay was developed for determination of E. coli O157:H7. This is based on an antimicrobial peptide-mediated nanocomposite pair and uses a personal glucose meter as signal readout. The antimicrobial peptides, magainins I, and cecropin P1 were employed as recognition molecules for the nanocomposite pair, respectively. With a one-step process, copper phosphate nanocomposites embedded by magainins I and Fe3O4 were used as “capturing” probes for bacterial magnetic isolation, and calcium phosphate nanocomplexes composed of cecropin P1 and invertase were used as signal tags. After magnetic separation, the invertase of the signal tags hydrolyzed sucrose to glucose, thereby converting E. coli O157:H7 levels to glucose levels. This latter can be quantified by a personal glucose meter. Under optimal conditions, the concentration of E. coli O157:H7 can be determined in a linear range of 10 to 107 CFU·mL−1 with a detection limit of 10 CFU·mL−1. The method was successfully applied to the determination of E. coli O157:H7 in milk samples.

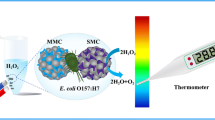
Schematic representation of sandwich immunoassay for E. coli O157:H7. One-pot synthetic of Fe3O4-magainins I nanocomposites (MMP) were used for magnetic capture. Cecropin P1-invertase nanocomposites (PIP) were used as signal tags. A personal glucose meter was used as readout to determine the target.




Similar content being viewed by others
References
Pop M, Walker AW, Paulson J, Lindsay B, Antonio M, Hossain MA, Oundo J, Tamboura B, Mai V, Astrovskaya I, Bravo HC, Rance R, Stares M, Levine MM, Panchalingam S, Kotloff K, Ikumapayi UN, Ebruke C, Adeyemi M, Ahmed D, Ahmed F, Alam MT, Amin R, Siddiqui S, Ochieng JB, Ouma E, Juma J, Mailu E, Omore R, Morris JG, Breiman RF, Saha D, Parkhill J, Nataro JP, Stine OC (2014) Diarrhea in young children from low-income countries leads to large-scale alterations in intestinal microbiota composition. Genome Biol 15(6):R76. https://doi.org/10.1186/gb-2014-15-6-r76
Lupindu AM (2018) Epidemiology of Shiga toxin-producing Escherichia coli O157:H7 in Africa in review. South Afr J Infect Dis 33(1):24–30. https://doi.org/10.1080/23120053.2017.1376558
Zhu G, Yan B, Xing M, Tian C (2018) Automated counting of bacterial colonies on agar plates based on images captured at near-infrared light. J Microbiol Methods 153:66–73. https://doi.org/10.1016/j.mimet.2018.09.004
Rusak LA, de Castro Lisboa Pereira R, Freitag IG, Hofer CB, Hofer E, Asensi MD, Vallim DC (2018) Rapid detection of Yersinia enterocolitica serotype O:3 using a duplex PCR assay. J Microbiol Methods 154:107–111. https://doi.org/10.1016/j.mimet.2018.10.014
Kumar BK, Raghunath P, Devegowda D, Deekshit VK, Venugopal MN, Karunasagar I, Karunasagar I (2011) Development of monoclonal antibody based sandwich ELISA for the rapid detection of pathogenic Vibrio parahaemolyticus in seafood. Int J Food Microbiol 145(1):244–249. https://doi.org/10.1016/j.ijfoodmicro.2010.12.030
Shahdordizadeh M, Taghdisi SM, Ansari N, Alebooye Langroodi F, Abnous K, Ramezani M (2017) Aptamer based biosensors for detection of Staphylococcus aureus. Sensors Actuators B Chem 241:619–635. https://doi.org/10.1016/j.snb.2016.10.088
Tao Y, Yang J, Chen L, Huang Y, Qiu B, Guo L, Lin Z (2018) Dialysis assisted ligand exchange on gold nanorods: amplification of the performance of a lateral flow immunoassay for E. coli O157:H7. Microchim Acta 185(7):350. https://doi.org/10.1007/s00604-018-2897-0
Majdinasab M, Hayat A, Marty JL (2018) Aptamer-based assays and aptasensors for detection of pathogenic bacteria in food samples. TrAC Trends Anal Chem 107:60–77. https://doi.org/10.1016/j.trac.2018.07.016
Park KS (2018) Nucleic acid aptamer-based methods for diagnosis of infections. Biosens Bioelectron 102:179–188. https://doi.org/10.1016/j.bios.2017.11.028
Karimzadeh A, Hasanzadeh M, Shadjou N, Guardia MDL (2018) Peptide based biosensors. TrAC Trends Anal Chem 107:1–20. https://doi.org/10.1016/j.trac.2018.07.018
Liu Q, Wang J, Boyd BJ (2015) Peptide-based biosensors. Talanta 136:114–127. https://doi.org/10.1016/j.talanta.2014.12.020
Shai Y (1999) Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta 1462(1–2):55–70. https://doi.org/10.1016/s0005-2736(99)00200-x
Kulagina NV, Shaffer KM, Ligler FS, Taitt CR (2007) Antimicrobial peptides as new recognition molecules for screening challenging species. Sensors Actuators B Chem 121(1):150–157. https://doi.org/10.1016/j.snb.2006.09.044
Liu X, Marrakchi M, Xu D, Dong H, Andreescu S (2016) Biosensors based on modularly designed synthetic peptides for recognition, detection and live/dead differentiation of pathogenic bacteria. Biosens Bioelectron 80:9–16. https://doi.org/10.1016/j.bios.2016.01.041
Zhou C, Zou H, Li M, Sun C, Ren D, Li Y (2018) Fiber optic surface plasmon resonance sensor for detection of E. coli O157:H7 based on antimicrobial peptides and AgNPs-rGO. Biosens Bioelectron 117:347–353. https://doi.org/10.1016/j.bios.2018.06.005
Akinloye O, Krishnamurthy R, Wishart D, Goss GG (2017) Peptide-based fluorescence biosensors for detection/measurement of nanoparticles. Anal Bioanal Chem 409(4):903–915. https://doi.org/10.1007/s00216-016-0042-7
Qiao Z, Lei C, Fu Y, Li Y (2017) An antimicrobial peptide-based colorimetric bioassay for rapid and sensitive detection of E-coli O157:H7. RSC Adv 7(26):15769–15775. https://doi.org/10.1039/c6ra28362d
de Miranda JL, Oliveira MDL, Oliveira IS, Frias IAM, Franco OL, Andrade CAS (2017) A simple nanostructured biosensor based on clavanin a antimicrobial peptide for gram-negative bacteria detection. Biochem Eng J 124:108–114. https://doi.org/10.1016/j.bej.2017.04.013
Lv E, Ding J, Qin W (2018) Potentiometric detection of listeria monocytogenes via a short antimicrobial peptide pair-based sandwich assay. Anal Chem 90(22):13600–13606. https://doi.org/10.1021/acs.analchem.8b03809
Kulagina NV, Lassman ME, Ligler FS, Taitt CR (2005) Antimicrobial peptides for detection of bacteria in biosensor assays. Anal Chem 77(19):6504–6508. https://doi.org/10.1021/ac050639r
Mannoor MS, Tao H, Clayton JD, Sengupta A, Kaplan DL, Naik RR, Verma N, Omenetto FG, McAlpine MC (2012) Graphene-based wireless bacteria detection on tooth enamel. Nat Commun 3:763. https://doi.org/10.1038/ncomms1767
Mannoor MS, Zhang S, Link AJ, McAlpine MC (2010) Electrical detection of pathogenic bacteria via immobilized antimicrobial peptides. Proc Natl Acad Sci 107(45):19207–19212. https://doi.org/10.1073/pnas.1008768107
Ariza-Avidad M, Salinas-Castillo A, Capitán-Vallvey LF (2016) A 3D μPAD based on a multi-enzyme organic–inorganic hybrid nanoflower reactor. Biosens Bioelectron 77:51–55. https://doi.org/10.1016/j.bios.2015.09.012
Wang LB, Wang Y-C, He R, Zhuang A, Wang X, Zeng J, Hou JG (2013) A new nanobiocatalytic system based on allosteric effect with dramatically enhanced enzymatic performance. J Am Chem Soc 135(4):1272–1275. https://doi.org/10.1021/ja3120136
Liu Y, Chen J, Du M, Wang X, Ji X, He Z (2017) The preparation of dual-functional hybrid nanoflower and its application in the ultrasensitive detection of disease-related biomarker. Biosens Bioelectron 92:68–73. https://doi.org/10.1016/j.bios.2017.02.004
Zhang B, Chen J, Wang J, Huyan Y, Zhang H, Zhang Q (2018) Flowerlike BSA/Zn3(PO4)2/Fe3O4 magnetic hybrid particles: preparation and application to adsorption of copper ions. J Chem Eng Data 63(10):3913–3922. https://doi.org/10.1021/acs.jced.8b00544
Ge J, Lei J, Zare RN (2012) Protein-inorganic hybrid nanoflowers. Nat Nanotechnol 7(7):428–432. https://doi.org/10.1038/nnano.2012.80
Ye R, Zhu C, Song Y, Song J, Fu S, Lu Q, Yang X, Zhu MJ, Du D, Li H, Lin Y (2016) One-pot bioinspired synthesis of all-inclusive protein-protein nanoflowers for point-of-care bioassay: detection of E. coli O157:H7 from milk. Nanoscale 8(45):18980–18986. https://doi.org/10.1039/c6nr06870g
Wei T, Du D, Zhu M-J, Lin Y, Dai Z (2016) An improved ultrasensitive enzyme-linked immunosorbent assay using hydrangea-like antibody-enzyme-inorganic three-in-one Nanocomposites. ACS Appl Mater Interfaces 8(10):6329–6335. https://doi.org/10.1021/acsami.5b11834
Wang K-Y, Bu S-J, Ju C-J, Li C-T, Li Z-Y, Han Y, Ma C-Y, Wang C-Y, Hao Z, Liu W-S, Wan J-Y (2018) Hemin-incorporated nanoflowers as enzyme mimics for colorimetric detection of foodborne pathogenic bacteria. Bioorg Med Chem Lett 28(23):3802–3807. https://doi.org/10.1016/j.bmcl.2018.07.017
Bu S-J, Wang K-Y, Bai H-S, Leng Y, Ju C-J, Wang C-Y, Liu W-S, Wan J-Y (2019) Immunoassay for pathogenic bacteria using platinum nanoparticles and a hand-held hydrogen detector as transducer. Application to the detection of Escherichia coli O157:H7. Microchim Acta 186 (5):296. https://doi.org/10.1007/s00604-019-3409-6
Gregory K, Mello CM (2005) Immobilization of Escherichia coli cells by use of the antimicrobial peptide cecropin P1. Appl Environ Microbiol 71(3):1130–1134. https://doi.org/10.1128/AEM.71.3.1130-1134.2005
Ourth DD, Lockey TD, Renis HE (1994) Induction of Cecropin-like and attacin-like antibacterial but not antiviral activity in Heliothis virescens larvae. Biochem Biophys Res Commun 200(1):35–44. https://doi.org/10.1006/bbrc.1994.1410
Zasloff M (1987) Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A 84(15):5449–5453. https://doi.org/10.1073/pnas.84.15.5449
Li Y, Afrasiabi R, Fathi F, Wang N, Xiang C, Love R, She Z, Kraatz H-B (2014) Impedance based detection of pathogenic E. coli O157:H7 using a ferrocene-antimicrobial peptide modified biosensor. Biosens Bioelectron 58:193–199. https://doi.org/10.1016/j.bios.2014.02.045
Funding
This work was financially supported by the National Key Research and Development Program of China (No. 2016YFD0501001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM1
(DOCX 458 kb)
Rights and permissions
About this article
Cite this article
Bai, H., Bu, S., Wang, C. et al. Sandwich immunoassay based on antimicrobial peptide-mediated nanocomposite pair for determination of Escherichia coli O157:H7 using personal glucose meter as readout. Microchim Acta 187, 220 (2020). https://doi.org/10.1007/s00604-020-4200-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-4200-4