Abstract
Aptamers against deoxynivalenol (DON) were selected through capture-systematic evolution of ligands by exponential enrichment. Through isothermal titration calorimetry and fluorimetric assay, aptamer candidate DN-2 demonstrated good affinity to DON with Kd value of 40.36 ± 6.32 nM. Accordingly, a Forster resonance energy transfer aptasensor was fabricated by using the aptamer DN-2 combined with AuCu bimetallic nanoclusters as energy donor and MoS2 nanosheets as energy acceptor. Under the optimal conditions, the fluorescence response was utilized for DON quantitative determination ranging from 5 to 100 ng/mL with a detection limit of 1.87 ng/mL. The practical application of this method was verified in maize flour samples and demonstrated a satisfied recovery of 94.6 ~ 103.1%. The obtained aptamers and their application in DON determination provide a new tool for DON monitoring in various foodstuff.
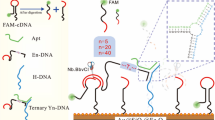
Graphical abstract






Similar content being viewed by others
References
Xu L, Zhang Z, Zhang QZ, Li P (2016) Mycotoxin determination in foods using advanced sensors based on antibodies or aptamers. Toxins 8:239
Cortinovis C, Battini M, Caloni F (2012) Deoxynivalenol and T-2 toxin in raw feeds for horses. J Equine Vet Sci 32:72–74
Pestka JJ (2010) Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch Toxicol 84:663–679
Kouadio JH, Dano SD, Moukha S, Mobio TA, Creppy EE (2007) Effects of combinations of Fusarium mycotoxins on the inhibition of macromolecular synthesis, malondialdehyde levels, DNA methylation and fragmentation, and viability in Caco-2 cells. Toxicon 49:306–317
Song SQ, Liu N, Zhao ZY, Njumbe EE, Wu S, Sun C, Wu A (2014) Multiplex lateral flow immunoassay for mycotoxin determination. Anal Chem 86:4995–5001
Ji F, Xu J, Liu X, Yin X, Shi J (2014) Natural occurrence of deoxynivalenol and zearalenone in wheat from Jiangsu province. China Food Chem 157:393–397
Belajova E, Rauova D (2008) Application of a simple and rapid pre-treatment procedure in the high performance liquid chromatographic analysis of deoxynivalenol and zearalenone in beer. J Food Nutr Res 47:189–199
Sui K, Li J, Feng W, Zhao SC (2005) Determination of deoxynivalenol in cereal grains by high performance liquid chromatography and verified by high performance liquid chromatography-mass spectrometry. Chin J Anal Chem 33:1643–1646
Sanders M, De Boevre M, Dumoulin F, Detavernier CL, Martens F, Van Poucke C, Eeckhout M, Eeckhout S, De Saeger S (2013) Sampling of wheat dust and subsequent analysis of deoxynivalenol by LC-MS/MS. J Agric Food Chem 61:6259–6264
Schneider L, Pichler H, Krska R (2000) An enzyme linked immunoassay for the determination of deoxynivalenol in wheat based on chicken egg yolk antibodies. Fresenius J Anal Chem 367:98–100
Maragos CM, Plattner RD (2002) Rapid fluorescence polarization immunoassay for the mycotoxin deoxynivalenol in wheat. J Agric Food Chem 50:1827–1832
Valenzano S, Lippolis V, Pascale M, De Marco A, Maragos CM, Suman M, Visconti A (2014) Determination of deoxynivalenol in wheat bran and whole-wheat flour by fluorescence polarization immunoassay. Food Anal Meth 7:806–813
Yu Q, Li H, Li C, Zhang S, Shen J, Wang Z (2015) Gold nanoparticles-based lateral flow immunoassay with silver staining for simultaneous detection of fumonisin B1 and deoxynivalenol. Food Control 54:347–352
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346:818–822
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249:505–510
Ni S, Yao H, Wang L, Lu J, Jiang F, Lu A, Zhang G (2017) Chemical modifications of nucleic acid aptamers for therapeutic purposes. Int J Mol Sci 18:1683
Zhou J, Rossi J (2017) Aptamers as targeted therapeutics: current potential and challenges. Nat Rev Drug Discov 16:181–202
Jin R, Zeng C, Zhou M, Chen Y (2016) Atomically precise colloidal metal nanoclusters and nanoparticles: fundamentals and opportunities. Chem Rev 116:10346–10413
Li D, Kumari B, Makabenta JM, Gupta A, Rotello V (2019) Effective detection of bacteria using metal nanoclusters. Nanoscale 11:22172–22181
Wang DS, Li Y (2011) Bimetallic nanocrystals: liquid-phase synthesis and catalytic applications. Adv Mater 23:1044–1060
Zhai QF, Xing HH, Fan DQ, Zhang XW, Li J, Wang E (2018) Gold-silver bimetallic nanoclusters with enhanced fluorescence for highly selective and sensitive detection of glutathione. Sens Actuators B Chem 273:1827–1832
Xu N, Meng L, Li HW, Lu DY, Wu YQ (2018) Polyethyleneimine capped bimetallic Au/Pt nanoclusters are a viable fluorescent probe for specific recognition of chlortetracycline among other tetracycline antibiotics. Microchim Acta 185:1436–5073
Ge J, Ou EC, Yu RQ, Chu X (2014) A novel aptameric nanobiosensor based on theself-assembled DNA-MoS2 nanosheet architecture for biomolecule detection. J Mater Chem B 2:625–628
Kong RM, Ding L, Wang ZJ, You JM, Qu FL (2015) A novel aptamer-functionalized MoS2 nanosheet fluorescent biosensor for sensitive detection of prostate specific antigen. Anal Bioanal Chem 407:369–377
Xu Y, Kang Q, Yang B, Chen BB, He M, Hu B (2020) A nanoprobe based on molybdenum disulfide nanosheets and silver nanoclusters for imaging and quantification of intracellular adenosine triphosphate. Anal Chim Acta 1134:75–83
Yuan YX, Yu HC, Yin Y (2020) A highly sensitive aptasensor for vascular endothelial growth factor based on fluorescence resonance energy transfer from upconversion nanoparticles to MoS2 nanosheets. Anal Methods 12:4466–4472
Sameiyan E, Khoshbin Z, Lavaee P, Ramezani M, Alibolandi M, Abnous K, Taghdisi SM (2021) A bivalent binding aptamer-cDNA on MoS2 nanosheets based fluorescent aptasensor for detection of aflatoxin M1. Talanta 235:122779
Lin XF, Li CX, He CX, Zhou Y, Wang ZP, Duan N, Wu SJ (2021) Upconversion nanoparticles assembled with gold nanourchins as luminescence and surface-enhanced raman scattering dual-mode aptasensors for detection of ochratoxin A. ACS Appl Nano Mater 4:8231–8240
Wu SJ, Duan N, Ma XY, Xia Y, Wang HG, Wang ZP, Zhang Q (2012) Multiplexed fluorescence resonance energy transfer aptasensor between upconversion nanoparticles and graphene oxide for the simultaneous determination of mycotoxins. Anal Chem 84:6263–6270
Ong CC, Sangu SS, Illias NM, Gopinath SCB, Saheed MSM (2020) Iron nanoflorets on 3D-graphene-nickel: a ‘Dandelion’ nanostructure for selective deoxynivalenol detection. Biosens Bioelectron 154:112088
Subak H, Selvolini G, Macchiagodena M, Ozkan-Ariksoysal D, Pagliai M, Procacci P, Marrazza G (2021) Mycotoxins aptasensing: from molecular docking to electrochemical detection of deoxynivalenol. Bioelectrochemistry 138:107691
Duan N, Gong WH, Wu SJ, Wang ZP (2017) An ssDNA library immobilized SELEX technique for selection of an aptamer against ractopamine. Anal Chim Acta 961:100–105
Shojaeifard Z, Heidari N, Hemmateenejad B (2019) Bimetallic AuCu nanoclusters-based florescent chemosensor for sensitive detection of Fe3+ in environmental and biological systems. Spectrochim Acta A 209:202–208
Curtis Johnson W Jr (1996) Determination of the conformation of nucleic acid by electronic CD. New York. pp 433–468
Ratmeyer L, Vinayak R, Zhong YY, Zon G, Wilson WD (1994) Sequence specific thermodynamic and structural properties for DNA RNA duplexes. Biochemistry 33:5298–5304
Chen ZC, Ding WH, Gu YY, Gao S, Yun DM, Wang CN, Li WQ, Sun F (2018) Dopamine-modified AuCu bimetallic nanoclusters as charge transfer-based biosensors for highly sensitive glycine detection. Langmuir 36:13928–13936
Liu LF, Mu XY, Liu HX, Wang Q, Bai XT, Wang JY, Liu HL, Xu FJ, Jing YQ, Dai HT, Liu CL, He H, Zhang XD (2018) Structure, luminescence, and bioimaging of bimetallic CuAu nanoclusters. Opt Mater 86:291–297
Funding
This work was partially supported by the National Natural Science Fund of China (NSFC 32072310, 31871721), the Guangzhou Science and Technology Project (202206010096), the Project funded by Jiangsu Province Postdoctoral Science Foundation (1701097B), and the Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duan, N., Li, C., Song, M. et al. Deoxynivalenol fluorescence aptasensor based on AuCu bimetallic nanoclusters and MoS2. Microchim Acta 189, 296 (2022). https://doi.org/10.1007/s00604-022-05385-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05385-4