Abstract
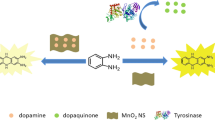
A self-correcting fluorescent assay of tyrosinase (TYR) was developed by utilization of Fe-MIL-88B-NH2 as a peroxidase-like nanozyme and a capture probe. Fe-MIL-88B-NH2 nanozyme was selected as an electron donor, and the oxidization product (dopamine-o-quinone) acts as an energy acceptor. First, TYR catalyzes the oxidation of tyramine hydrochloride to dopamine and then to dopamine-o-quinone. Second, Fe-MIL-88B-NH2 with intrinsic peroxidase-like activity decomposes H2O2 to produce ·OH radicals, which further accelerate the oxidation of dopamine to dopamine-o-quinone. Excessive H2O2 and ·OH radicals reduce the interferences from ascorbic acid at the same time providing a self-correcting ability. Dopamine-o-quinone reacts with -NH2 groups on the ligand of Fe-MIL-88B-NH2 through Michael reaction which results in fluorescence quenching. Under 365-nm excitation, the fluorescence emission intensity at 452 nm gradually decreased with increasing TYR concentration varying from 0 to 10 U mL−1. The linear range is from 1 to 5 U mL−1 and the detection limit is 0.05679 U mL−1. This self-correcting fluorescent assay of tyrosinase exhibits good sensitivity and selectivity which is also successfully applied for tyrosinase inhibitor detection.
Graphical abstract

Schematic representation of fluorescent assay for tyrosinase determination based on Fe-MIL-88B-NH2 nanozyme.
A self-correcting fluorescent assay for tyrosinase was developed based on the Fe-MIL-88B-NH2 nanozyme.







Similar content being viewed by others
References
Zhu X, Hu J, Zhao Z, Sun M, Chi X, Wang X, Gao J (2015) Kinetic and sensitive analysis of tyrosinase activity using electron transfer complexes: in vitro and intracellular study. Small 11(7):862–870. https://doi.org/10.1002/smll.201401595
Solem E, Tuczek F, Decker H (2016) Tyrosinase versus catechol oxidase: one asparagine makes the difference. Angew Chem Int Ed 55(8):2884–2888. https://doi.org/10.1002/anie.201508534
Li S, Hu R, Wang S, Guo X, Zeng Y, Li Y, Yang G (2018) Specific imaging of tyrosinase in vivo with 3-hydroxybenzyl caged D-luciferins. Anal Chem 90(15):9296–9300. https://doi.org/10.1021/acs.analchem.8b01874
Chiang L, Keown W, Citek C, Wasinger EC, Stack TD (2016) Simplest monodentate imidazole stabilization of the oxy-tyrosinase Cu2O2 core: phenolate hydroxylation through a cu(III) intermediate. Angew Chem Int Ed 55(35):10453–10457. https://doi.org/10.1002/anie.201605159
Zhao J, Bao X, Wang S, Lu S, Sun J, Yang X (2017) In situ fluorogenic and chromogenic reactions for the sensitive dual-readout assay of tyrosinase activity. Anal Chem 89(19):10529–10536. https://doi.org/10.1021/acs.analchem.7b02739
Zhou J, Shi W, Li L, Gong Q, Wu X, Li X, Ma H (2016) Detection of misdistribution of tyrosinase from melanosomes to lysosomes and its upregulation under psoralen/ultraviolet a with a melanosome-targeting tyrosinase fluorescent probe. Anal Chem 88(8):4557–4564. https://doi.org/10.1021/acs.analchem.6b00742
Yan X, Li H, Zheng W, Su X (2015) Visual and fluorescent detection of tyrosinase activity by using a dual-emission ratiometric fluorescence probe. Anal Chem 87(17):8904–8909. https://doi.org/10.1021/acs.analchem.5b02037
Chai L, Zhou J, Feng H, Tang C, Huang Y, Qian Z (2015) Functionalized carbon quantum dots with dopamine for tyrosinase activity monitoring and inhibitor screening: in vitro and intracellular investigation. ACS Appl Mater Interfaces 7(42):23564–23574. https://doi.org/10.1021/acsami.5b06711
Qu Z, Yu T, Bi L (2019) A dual-channel ratiometric fluorescent probe for determination of the activity of tyrosinase using nitrogen-doped graphene quantum dots and dopamine-modified CdTe quantum dots. Mikrochim Acta 186(9):635–643. https://doi.org/10.1007/s00604-019-3733-x
Teng Y, Jia X, Li J, Wang E (2015) Ratiometric fluorescence detection of tyrosinase activity and dopamine using thiolate-protected gold nanoclusters. Anal Chem 87(9):4897–4902. https://doi.org/10.1021/acs.analchem.5b00468
Hashemi SA, Mousavi SM, Bahrani S, Ramakrishna S, Babapoor A, Chiang WH (2020) Coupled graphene oxide with hybrid metallic nanoparticles as potential electrochemical biosensors for precise detection of ascorbic acid within blood. Anal Chim Acta 1107:183–192. https://doi.org/10.1016/j.aca.2020.02.018
Silveira-Dorta G, Monzon DM, Crisostomo FP, Martin T, Martin VS, Carrillo R (2015) Oxidation with air by ascorbate-driven quinone redox cycling. Chem Commun 51(32):7027–7030. https://doi.org/10.1039/c5cc01519g
Ma W, Long YT (2014) Quinone/hydroquinone-functionalized biointerfaces for biological applications from the macro- to nano-scale. Chem Soc Rev 43(1):30–41. https://doi.org/10.1039/c3cs60174a
Zhao J, Liu G, Sun J, Wang Q, Li ZJ, Yang X (2020) Dual-readout tyrosinase activity assay facilitated by a chromo-fluorogenic reaction between catechols and naphthoresorcin. Anal Chem 92(2):2316–2322. https://doi.org/10.1021/acs.analchem.9b05204
Niu X, Li X, Lyu Z, Pan J, Ding S, Ruan X, Zhu W, Du D, Lin Y (2020) Metal-organic framework based nanozymes: promising materials for biochemical analysis. Chem Commun 56(77):11338–11353. https://doi.org/10.1039/d0cc04890a
Jiang H, Jia J, Shkurenko A, Chen Z, Adil K, Belmabkhout Y, Weselinski LJ, Assen AH, Xue DX, O'Keeffe M, Eddaoudi M (2018) Enriching the reticular chemistry repertoire: merged nets approach for the rational design of intricate mixed-linker metal-organic framework platforms. J Am Chem Soc 140(28):8858–8867. https://doi.org/10.1021/jacs.8b04745
Chen Y-Z, Zhang R, Jiao L, Jiang H-L (2018) Metal–organic framework-derived porous materials for catalysis. Coord Chem Rev 362:1–23. https://doi.org/10.1016/j.ccr.2018.02.008
Mendes RF, Figueira F, Leite JP, Gales L, Almeida Paz FA (2020) Metal-organic frameworks: a future toolbox for biomedicine? Chem Soc Rev 49(24):9121–9153. https://doi.org/10.1039/d0cs00883d
Kang Y-S, Lu Y, Chen K, Zhao Y, Wang P, Sun W-Y (2019) Metal–organic frameworks with catalytic centers: from synthesis to catalytic application. Coord Chem Rev 378:262–280. https://doi.org/10.1016/j.ccr.2018.02.009
Li H, Li L, Lin R-B, Zhou W, Zhang Z, Xiang S, Chen B (2019) Porous metal-organic frameworks for gas storage and separation: status and challenges. Energy Chem 1(1):100006. https://doi.org/10.1016/j.enchem.2019.100006
Ren R, Cai G, Yu Z, Zeng Y, Tang D (2018) Metal-polydopamine framework: an innovative signal-generation tag for colorimetric immunoassay. Anal Chem 90(18):11099–11105. https://doi.org/10.1021/acs.analchem.8b03538
Lv S, Zhang K, Zhu L, Tang D, Niessner R, Knopp D (2019) H2-based electrochemical biosensor with Pd nanowires@ZIF-67 molecular sieve bilayered sensing Interface for immunoassay. Anal Chem 91(18):12055–12062. https://doi.org/10.1021/acs.analchem.9b03177
Lv S, Zhang K, Zhu L, Tang D (2020) ZIF-8-assisted NaYF4:Yb,Tm@ZnO converter with exonuclease III-powered DNA walker for near-infrared light responsive biosensor. Anal Chem 92(1):1470–1476. https://doi.org/10.1021/acs.analchem.9b04710
Cai W, Gao H, Chu C, Wang X, Wang J, Zhang P, Lin G, Li W, Liu G, Chen X (2017) Engineering phototheranostic nanoscale metal-organic frameworks for multimodal imaging-guided cancer therapy. ACS Appl Mater Interfaces 9(3):2040–2051. https://doi.org/10.1021/acsami.6b11579
Huang Y, Ren J, Qu X (2019) Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem Rev 119(6):4357–4412. https://doi.org/10.1021/acs.chemrev.8b00672
Liang M, Yan X (2019) Nanozymes: from new concepts, mechanisms, and standards to applications. Acc Chem Res 52(8):2190–2200. https://doi.org/10.1021/acs.accounts.9b00140
Wu J, Wang X, Wang Q, Lou Z, Li S, Zhu Y, Qin L, Wei H (2019) Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem Soc Rev 48(4):1004–1076. https://doi.org/10.1039/c8cs00457a
Yu Z, Cai G, Liu X, Tang D (2020) Platinum nanozyme-triggered pressure-based immunoassay using a three-dimensional polypyrrole foam-based flexible pressure sensor. ACS Appl Mater Interfaces 12(36):40133–40140. https://doi.org/10.1021/acsami.0c12074
Zeng R, Luo Z, Zhang L, Tang D (2018) Platinum nanozyme-catalyzed gas generation for pressure-based bioassay using polyaniline nanowires-functionalized graphene oxide framework. Anal Chem 90(20):12299–12306. https://doi.org/10.1021/acs.analchem.8b03889
Hou L, Qin Y, Li J, Qin S, Huang Y, Lin T, Guo L, Ye F, Zhao S (2019) A ratiometric multicolor fluorescence biosensor for visual detection of alkaline phosphatase activity via a smartphone. Biosens Bioelectron 143:111605. https://doi.org/10.1016/j.bios.2019.111605
Han LJ, Kong YJ, Hou GZ, Chen HC, Zhang XM, Zheng HG (2020) A europium-based MOF fluorescent probe for efficiently detecting malachite green and uric acid. Inorg Chem 59(10):7181–7187. https://doi.org/10.1021/acs.inorgchem.0c00620
Li Y, Guo A, Chang L, Li W-J, Ruan W-J (2017) Luminescent metal-organic-framework-based label-free assay of polyphenol oxidase with fluorescent scan. Chem Eur J 23(27):6562–6569. https://doi.org/10.1002/chem.201605992
Hou L, Qin Y, Lin T, Sun Y, Ye F, Zhao S (2020) Michael reaction-assisted fluorescent sensor for selective and one step determination of catechol via bifunctional Fe-MIL-88NH2 nanozyme. Sensors Actuators B Chem 321:128547–128553. https://doi.org/10.1016/j.snb.2020.128547
Bauer S, Serre C, Devic T, Horcajada P, Marrot J, Ferey G, Stock N (2008) High-throughput assisted rationalization of the formation of metal organic frameworks in the Iron(III) aminoterephthalate solvothermal system. Inorg Chem 47(17):7568–7576. https://doi.org/10.1021/ic800538r
Ma M, Bétard A, Weber I, Al-Hokbany NS, Fischer RA, Metzler-Nolte N (2013) Iron-based metal–organic frameworks MIL-88B and NH2-MIL-88B: high quality microwave synthesis and solvent-induced lattice “breathing”. Cryst Growth Des 13(6):2286–2291. https://doi.org/10.1021/cg301738p
Shi L, Wang T, Zhang H, Chang K, Meng X, Liu H, Ye J (2015) An amine-functionalized Iron(III) metal-organic framework as efficient visible-light photocatalyst for Cr(VI) reduction. Adv Sci 2(3):1500006. https://doi.org/10.1002/advs.201500006
Yi X, He X, Yin F, Yang T, Chen B, Li G (2020) NH2–MIL-88B–Fe for electrocatalytic N2 fixation to NH3 with high faradaic efficiency under ambient conditions in neutral electrolyte. J Mater Sci 55(26):12041–12052. https://doi.org/10.1007/s10853-020-04777-2
Wang D, Chen C, Ke X, Kang N, Shen Y, Liu Y, Zhou X, Wang H, Chen C, Ren L (2015) Bioinspired near-infrared-excited sensing platform for in vitro antioxidant capacity assay based on upconversion nanoparticles and a dopamine-melanin hybrid system. ACS Appl Mater Interfaces 7(5):3030–3040. https://doi.org/10.1021/am5086269
Funding
The authors are grateful for the financial support from the National Natural Science Foundation of China (21765002), the Natural Science Foundation of Guangxi (AD19110004, 2017GXNSFDA198044), and the BAGUI Scholar Program.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 5854 kb)
Rights and permissions
About this article
Cite this article
Sun, Y., Lin, T., Zeng, C. et al. A self-correcting fluorescent assay of tyrosinase based on Fe-MIL-88B-NH2 nanozyme. Microchim Acta 188, 158 (2021). https://doi.org/10.1007/s00604-021-04808-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04808-y