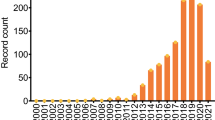
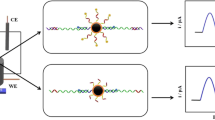
Abstract
The biology of the late twentieth century was marked by the discovery in 1993 of a new class of small non-coding ribonucleic acids (RNAs) which play major roles in regulating the translation and degradation of messenger RNAs. These small RNAs (18–25 nucleotides), called microRNAs (miRNAs), are implied in several biological processes such as differentiation, metabolic homeostasis, or cellular apoptosis and proliferation. The discovery in 2008 that the presence of miRNAs in body fluids could be correlated with cancer (prostate, breast, colon, lung, etc.) or other diseases (diabetes, heart diseases, etc.) has made them new key players as biomarkers. Therefore, miRNA detection is of considerable significance in both disease diagnosis and in the study of miRNA function. Until these days, more than 1200 miRNAs have been identified. However, traditional methods developed for conventional DNA does not apply satisfactorily for miRNA, in particular due to the low expression level of these miRNA in biofluids, and because they are very short strands. Electrochemical biosensors can provide this sensitivity and also offer the advantages of mass fabrication, low-cost, and potential decentralized analysis, which has wide application for microRNAs sensing, with many promising results already reported. The present review summarizes some newly developed electrochemical miRNA detection methods.








Similar content being viewed by others
References
Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75(5):843–854. https://doi.org/10.1016/0092-8674(93)90529-Y
Quintana ML, Rauhut R, Lendeckel W, Tuschl T (2001) Identification of novel genes coding for small expressed RNAs. Science 294(5543):853–885. https://doi.org/10.1126/science.1064921
Lee RC, Ambros V (2001) An extensive class of small RNAs in Caenorhabditis elegans. Science 294(5543):862–864. https://doi.org/10.1126/science.1065329
Ye J, Xu M, Tian X, Cai S, Zeng S (2019) Research advances in the detection of miRNA. J Pharm Anal 9(4):217–226. https://doi.org/10.1016/j.jpha.2019.05.004
de Planell-Saguer M, Rodicio MC (2011) Analytical aspects of microRNA in diagnostics: a review. Anal Chim Acta 699(2):134–152. https://doi.org/10.1016/j.aca.2011.05.025
Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ (2008) miRBase: tools for microRNA genomics. Nucleic Acids Res 36:154–158. https://doi.org/10.1093/nar/gkm952
Lautner G, Gyurcsányi RE (2014) Electrochemical detection of miRNAs. Electroanalysis 26(6 Special Issue: Electroanalysis-Based Clinical Diagnostics):1224–1235. https://doi.org/10.1002/elan.201400055
Masud MK, Umer M, Hossain SA, Yamauchi Y, Nguyen N-T, Shiddiky MJA (2019) Nanoarchitecture frameworks for electrochemical miRNA detection. Trends Biochem Sci 44(5):433–452. https://doi.org/10.1016/j.tibs.2018.11.012
Brase JC, Wuttig D, Kuner R, Sültmann H (2010) Serum microRNAs as non-invasive biomarkers for cancer. Mol Cancer 9:306. https://doi.org/10.1186/1476-4598-9-306
Jamali AA, Pourhassan-Moghaddam M, Dolatabadi JEN, Omidi Y (2014) Nanomaterials on the road to microRNA detection with optical and electrochemical nanobiosensors. TrAC Trends Anal Chem 55:24–42. https://doi.org/10.1016/j.trac.2013.10.008
Wittmann J, Jäck HM (2010) Serum microRNAs as powerful cancer biomarkers. Biochim Biophys Acta 1806:200–207. https://doi.org/10.1016/j.bbcan.2010.07.002
Mujica ML, Gallay PA, Perrachione F, Montemerlo AE, Tamborelli LA, Vaschetti VM, Reartes DF, Bollo S, Rodríguez MC, Dalmasso PR, Rubianes MD, Rivas GA (2020) New trends in the development of electrochemical biosensors for the quantification of microRNAs. J Pharm Biomed Anal 189:113478. https://doi.org/10.1016/j.jpba.2020.113478
Wark AW, Lee HJ, Corn RM (2008) Multiplexed detection methods for profiling microRNA expression in biological samples. Angew Chem Int Ed 47:644–652. https://doi.org/10.1002/anie.200702450
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297. https://doi.org/10.1016/s0092-8674(04)00045-5
Dong H, Lei J, Ding L, Wen Y, Ju H, Zhang X (2013) MicroRNA: function, detection, and bioanalysis. Chem Rev 113(8):6207–6233. https://doi.org/10.1021/cr300362f
Cissell KA, Shrestha S, Deo SK (2007) MicroRNA detection: challenges for the analytical chemist. Anal Chem 79(13):4754–4761. https://doi.org/10.1021/ac051726m
Qavi AJ, Kindt JT, Bailey RC (2010) Sizing up the future of microRNA analysis. Anal Bioanal Chem 398(6):2535–2549. https://doi.org/10.1007/s00216-010-4018-8
Lim LP, Lau NC, Weinstein EG, Abdelhakim A, Yekta S, Rhoades MW, Burge CB, Bartel DP (2003) The microRNAs of Caenorhabditis elegans. Genes Dev 17:991–1008. https://doi.org/10.1101/gad.1074403
Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M, Lin C, Socci ND, Hermida L, Fulci V, Chiaretti S, Foà R, Schliwka J, Fuchs U, Novosel A, Müller RU (2007) A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 129(7):1401–1414. https://doi.org/10.1016/j.cell.2007.04.040
Berezikov E, van Tetering G, Verheul M, van de Belt J, van Laake L, Vos J, Verloop R, van de Wetering M, Guryev V, Takada S, van Zonneveld AJ, Mano H, Plasterk R, Cuppen E (2006) Many novel mammalian microRNA candidates identified by extensive cloning and RAKE analysis. Genome Res 16(10):1289–1298. https://doi.org/10.1101/gr.5159906
Sempere LF, Freemantle S, Rowe IP, Moss E, Dmitrovsky E, Ambros V (2004) Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol 5:R13. https://doi.org/10.1186/gb-2004-5-3-r13
Li W, Ruan K (2009) MicroRNA detection by microarray. Anal Bioanal Chem 394(4):1117–1124. https://doi.org/10.1007/s00216-008-2570-2
Baskerville S, Bartel DP (2005) Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA 11:241–247. https://doi.org/10.1261/rna.7240905
Raymond CK, Roberts BS, Garrett-Engele P, Lim LP, Johnson JM (2005) Simple, quantitative primer-extension PCR assay for direct monitoring of microRNAs and short-interfering RNAs. RNA 11(11):1737–1744. https://doi.org/10.1261/rna.2148705
Schmittgen TD, Jiang J, Liu Q, Yang L (2004) A high-throughput method to monitor the expression of microRNA precursors. Nucleic Acids Res 32(4):43e–443e. https://doi.org/10.1093/nar/gnh040
Jiang J, Lee EJ, Gusev Y, Schmittgen TD (2005) Real-time expression profiling of microRNA precursors in human cancer cell lines. Nucleic Acids Res 33(17):5394–5403. https://doi.org/10.1093/nar/gki863
Wienholds E, Kloosterman WP, Miska E, Alvarez-Saavedra E, Berezikov E, de Bruijn E, Horvitz HR, Kauppinen S, Plasterk RH (2005) MicroRNA expression in zebrafish embryonic development. Science 309(5732):310–311. https://doi.org/10.1126/science.1114519
Kloosterman WP, Wienholds E, de Bruijn E, Kauppinen S, Plasterk RHA (2006) In situ detection of miRNAs in animal embryos using LNA-modified oligonucleotide probes. Nat Methods 3:27–29. https://doi.org/10.1038/nmeth843
Creighton CJ, Reid JG, Gunaratne PH (2009) Expression profiling of microRNAs by deep sequencing. Brief Bioinform 10(5):490–497. https://doi.org/10.1093/bib/bbp019
Hunt EA, Goulding AM, Deo SK (2009) Direct detection and quantification of microRNAs. Anal Biochem 387(1):1–12. https://doi.org/10.1016/j.ab.2009.01.011
Válóczi A, Hornyik C, Varga N, Burgyán J, Kauppinen S, Havelda Z (2004) Sensitive and specific detection of microRNAs by northern blot analysis using LNA-modified oligonucleotide probes. Nucleic Acids Res 32(22):e175. https://doi.org/10.1093/nar/gnh171
Ramkissoon SH, Mainwaring LA, Sloand EM, Young NS, Kajigaya S (2006) Nonisotopic detection of microRNA using digoxigenin labeled RNA probes. Mol Cell Probes 20(1):1–4. https://doi.org/10.1016/j.mcp.2005.07.004
Balcells I, Cirera S, Busk PK (2011) Specific and sensitive quantitative RT-PCR of miRNAs with DNA primers. BMC Biotechnol 11:70. https://doi.org/10.1186/1472-6750-11-70
Shingara J, Keiger K, Shelton J, Laosinchai-Wolf W, Powers P, Conrad R, Brown D, Labourier E (2005) An optimized isolation and labeling platform for accurate microRNA expression profiling. RNA 11(9):1461–1470. https://doi.org/10.1261/rna.2610405
Nasheri N, Cheng J, Singaravelu R, Wu P, McDermott MT, Pezacki JP (2011) An enzyme-linked assay for the rapid quantification of microRNAs based on the viral suppressor of RNA silencing protein p19. Anal Biochem 412(2):165–172. https://doi.org/10.1016/j.ab.2011.01.030
Fang S, Lee HJ, Wark AW, Corn RM (2006) Attomole microarray detection of microRNAs by nanoparticle-amplified SPR imaging measurements of surface polyadenylation reactions. J Am Chem Soc 128(43):14044–14046. https://doi.org/10.1021/ja065223p
Jurinke C, Oeth P, Boom DVD (2004) MALDI-TOF mass spectrometry. Mol Biotechnol 26(2):147–163. https://doi.org/10.1385/MB:26:2:147
Joyner JC, Keuper KD, Cowan JA (2013) Analysis of RNA cleavage by MALDI-TOF mass spectrometry. Nucleic Acids Res 41(1):e3. https://doi.org/10.1093/nar/gks811
Goldman JM, Zhang LA, Manna A, Armitage BA, Ly DH, Schneider JW (2013) High affinity γPNA sandwich hybridization assay for rapid detection of short nucleic acid targets with single mismatch discrimination. Biomacromolecules 14(7):2253–2261. https://doi.org/10.1021/bm400388a
Katoh T, Sakaguchi Y, Miyauchi K, Suzuki T, Kashiwabara S, Baba T, Suzuki T (2009) Selective stabilization of mammalian microRNAs by 3′ adenylation mediated by the cytoplasmic poly(A) polymerase GLD-2. Genes Dev 23:433–438. https://doi.org/10.1101/gad.1761509
Chen QR, Yu LR, Tsang P, Wei JS, Song YK, Cheuk A, Chung JY, Hewitt SM, Veenstra TD, Khan J (2011) Systematic proteome analysis identifies transcription factor YY1 as a direct target of miR-34a. J Proteome Res 10(2):479–487. https://doi.org/10.1021/pr1006697
Sípová H, Zhang S, Dudley AM, Galas D, Wang K, Homola J (2010) Surface plasmon resonance biosensor for rapid label-free detection of microribonucleic acid at subfemtomole level. Anal Chem 82(24):10110–10115. https://doi.org/10.1021/ac102131s
Zhou WJ, Chen Y, Corn RM (2011) Ultrasensitive microarray detection of short RNA sequences with enzymatically modified nanoparticles and surface plasmon resonance imaging measurements. Anal Chem 83:3897–3902. https://doi.org/10.1021/ac200422u
Driskell JD, Seto AG, Jones LP, Jokela S, Dluhy RA, Zhao YP, Tripp RA (2008) Rapid microRNA (miRNA) detection and classification via surface-enhanced Raman spectroscopy (SERS). Biosens Bioelectron 24(4):917–922. https://doi.org/10.1016/j.bios.2008.07.060
Driskell JD, Tripp RA (2010) Label-free SERS detection of microRNA based on affinity for an unmodified silver nanorod array substrate. J Artic Chem Commun 46:3298–3300. https://doi.org/10.1039/C002059A
Drummond TG, Hill MG, Barton JK (2003) Electrochemical DNA sensors. Nat Biotechnol 21:1192–1199. https://doi.org/10.1038/nbt873
Gao Z, Yu YH (2007) Direct labeling microRNA with an electrocatalytic moiety and its application in ultrasensitive microRNA assays. Biosens Bioelectron 22:933–940. https://doi.org/10.1016/j.bios.2006.04.020
Gao Z, Yang Z (2006) Detection of microRNAs using electrocatalytic nanoparticle tags. Anal Chem 78:1470–1477. https://doi.org/10.1021/ac051726m
Peng YF, Gao ZQ (2011) Amplified detection of microRNA based on ruthenium oxide nanoparticle-initiated deposition of an insulating film. Anal Chem 83(3):820–827. https://doi.org/10.1021/ac102370s
Pöhlmann C, Sprinzl M (2010) Electrochemical detection of microRNAs via gap hybridization assay. Anal Chem 82(11):4434–4440. https://doi.org/10.1021/ac100186p
Wang T, Viennois E, Merlin D, Wang G (2015) Microelectrode miRNA sensors enabled by enzymeless electrochemical signal amplification. Anal Chem 87:8173–8180. https://doi.org/10.1021/acs.analchem.5b00780
Liu S, Yang Z, Chang Y, Chai Y, Yuan R (2018) An enzyme-free electrochemical biosensor combining target recycling with Fe3O4/CeO2@Au nanocatalysts for microRNA-21 detection. Biosens Bioelectron 119:170–175. https://doi.org/10.1016/j.bios.2018.08.006
Ren Y, Deng H, Shen W, Gao Z (2013) A highly sensitive and selective electrochemical biosensor for direct detection of microRNAs in serum. Anal Chem 85(9):4784–4789. https://doi.org/10.1021/ac400583e
Tian L, Qian K, Qi J, Liu Q, Yao C, Song W, Wang Y (2018) Gold nanoparticles superlattices assembly for electrochemical biosensor detection of microRNA-21. Biosens Bioelectron 99:564–570. https://doi.org/10.1016/j.bios.2017.08.035
Jolly P, Batistuti MR, Miodek A, Zhurauski P, Mulato M, Lindsay MA, Estrela P (2016) Highly sensitive dual mode electrochemical platform for microRNA detection. Sci Rep 6:36719. https://doi.org/10.1038/srep36719
Fan Y, Chen X, Trigg AD, Tung C, Kong J, Gao Z (2007) Detection of microRNAs using target-guided formation of conducting polymer nanowires in nanogaps. J Am Chem Soc 129(17):5437–5443. https://doi.org/10.1021/ja067477g
Gao Z, Deng H, Shen W, Ren Y (2013) A label-free biosensor for electrochemical detection of femtomolar microRNAs. Anal Chem 85(3):1624–1630. https://doi.org/10.1021/ac302883c
Fang Z, Kelley SO (2009) Direct Electrocatalytic mRNA detection using PNA-nanowire sensors. Anal Chem 81(2):612–617. https://doi.org/10.1021/ac801890f
Yang H, Hui A, Pampalakis G, Soleymani L, Liu FF, Sargent EH, Kelley SO (2009) Direct, electronic microRNA detection for the rapid determination of differential expression profiles. Angew Chem Int Ed 48:8461–8464. https://doi.org/10.1002/anie.200902577
Cosnier S (2003) Biosensors based on electropolymerized films: new trends. Anal Bioanal Chem 377(3):507–520. https://doi.org/10.1007/s00216-003-2131-7
Su S, Wu Y, Zhu D, Chao J, Liu X, Wan Y, Su Y, Zuo X, Fan C, Wang L (2016) On-electrode synthesis of shape-controlled hierarchical flower-like gold nanostructures for efficient interfacial DNA assembly and sensitive electrochemical sensing of microRNA. Small 12(28):3794–3801. https://doi.org/10.1002/smll.201601066
Zhou C, Cui K, Liu Y, Li L, Zhang L, Xu M, Ge S, Wang Y, Yu J (2020) Ultrasensitive lab-on-paper device via Cu/Co double-doped CeO2 nanospheres as signal amplifiers for electrochemical/visual sensing of miRNA-155. Sensors Actuators B Chem 321:128499. https://doi.org/10.1016/j.snb.2020.128499
Wang D, Hu L, Zhou H, Abdel-Halim ES, Zhu J-J (2013) Molecular beacon structure mediated rolling circle amplification for ultrasensitive electrochemical detection of microRNA based on quantum dots tagging. Electrochem Commun 33:80–83. https://doi.org/10.1016/j.elecom.2013.04.030
Moccia M, Caratelli V, Cinti S, Pede B, Avitabile C, Saviano M, LisaImbriani A, Moscone D, Arduini F (2020) Paper-based electrochemical peptide nucleic acid (PNA) biosensor for detection of miRNA-492: a pancreatic ductal adenocarcinoma biomarker. Biosens Bioelectron 165:112371. https://doi.org/10.1016/j.bios.2020.112371
Xu S, Chang Y, Wu Z, Li Y, Yuan R, Chai Y (2020) One DNA circle capture probe with multiple target recognition domains for simultaneous electrochemical detection of miRNA-21 and miRNA-155. Biosens Bioelectron 149:111848. https://doi.org/10.1016/j.bios.2019.111848
Kasturi S, Eom Y, Torati SR, Kim CG (2020) Highly sensitive electrochemical biosensor based on naturally reduced rGO/Au nanocomposite for the detection of miRNA-122 biomarker. J Ind Eng Chem 93:186–195. https://doi.org/10.1016/j.jiec.2020.09.022
Mandli J, Mohammadi H, Amine A (2017) Electrochemical DNA sandwich biosensor based on enzyme amplified microRNA-21 detection and gold nanoparticles. Bioelectrochemistry 116:17–23. https://doi.org/10.1016/j.bioelechem.2017.03.002
Han S, Liu W, Yang S, Wang R (2019) Facile and label-free electrochemical biosensors for microRNA detection based on DNA origami nanostructures. ACS Omega 4(6):11025–11031. https://doi.org/10.1021/acsomega.9b01166
Bharti A, Mittal S, Rana S, Dahiya D, Agnihotri N, Prabhakar N (2020) Electrochemical biosensor for miRNA-21 based on gold-platinum bimetallic nanoparticles coated 3-aminopropyltriethoxy silane. Anal Biochem 609:113908. https://doi.org/10.1016/j.ab.2020.113908
Lusi EA, Passamano M, Guarascio P, Scarpa A, Schiavo L (2009) Innovative electrochemical approach for an early detection of microRNAs. Anal Chem 81(7):2819–2822. https://doi.org/10.1021/ac8026788
Tran HV, Piro B, Reisberg S, Duc HT, Pham MC (2014) An innovative strategy for direct electrochemical detection of microRNAs biomarker. Anal Bioanal Chem 406:1241–1244. https://doi.org/10.1007/s00216-013-7292-4
Majd SM, Salimi A, Ghasemi F (2018) An ultrasensitive detection of miRNA-155 in breast cancer via direct hybridization assay using two-dimensional molybdenum disulfide field-effect transistor biosensor. Biosens Bioelectron 105:6–13. https://doi.org/10.1016/j.bios.2018.01.009
Zhang GJ, Chua JH, Chee RE, Agarwal A, Wong SM (2009) Label-free direct detection of MiRNAs with silicon nanowire biosensors. Biosens Bioelectron 24(8):2504–2508. https://doi.org/10.1016/j.bios.2008.12.035
Yaralı E, Kanat E, Erac Y, Erdem A (2019) Ionic liquid modified single-use electrode developed for voltammetric detection of miRNA-34a and its application to real samples. Electroanalysis 31:1–11. https://doi.org/10.1002/elan.201900353
Kilic T, Kaplan M, Demiroglu S, Erdem A, Ozsoze M (2016) Label-free electrochemical detection of microRNA-122 in real samples by graphene modified disposable electrodes. J Electrochem Soc 163(6):B227–B233. https://doi.org/10.1149/2.0481606jes
Pham MC, Hachemi A, Dubois JE (1984) An apparently totally electroactive polymer film obtained by electropolymerizing 5-hydroxy-1,4-naphthoquinone onto graphite. J Electroanal Chem Interfacial Electrochem 161(1):199–204. https://doi.org/10.1016/S0022-0728(84)80262-4
Audebert P, Bidan G (1987) Electrochemical study of poly(phas) in acetonitrile and water + acetonitrile electrolytes. J Electroanal Chem Interfacial Electrochem 238(1–2):183–195. https://doi.org/10.1016/0022-0728(87)85173-2
Piro B, Bazzaoui EA, Pham MC, Novak P, Haas O (1999) Multiple internal reflection FTIR spectroscopic (MIRFTIRS) study of the redox process of poly(5-amino-1,4-naphthoquinone) film in aqueous and organic media. Electrochim Acta 44(12):1953–1964. https://doi.org/10.1016/S0013-4686(98)00304-1
Reisberg S, Piro B, Noël V, Pham MC (2005) DNA electrochemical sensor based on conducting polymer: dependence of the “signal-on” detection on the probe sequence localization. Anal Chem 77(10):3351–3356. https://doi.org/10.1021/ac050080v
Tran HV, Yougnia R, Reisberg S, Piro B, Serradji N, Nguyen TD, Tran LD, Dong CZ, Pham MC (2012) A label-free electrochemical immunosensor for direct, signal-on and sensitive pesticide detection. Biosens Bioelectron 31(1):62–68. https://doi.org/10.1016/j.bios.2011.09.035
Zhang QD, March G, Noel V, Piro B, Reisberg S, Tran LD, Hai LV, Abadia E, Nielsen PE, Sola C, Pham MC (2012) Label-free and reagentless electrochemical detection of PCR fragments using self-assembled quinone derivative monolayer: application to Mycobacterium tuberculosis. Biosens Bioelectron 32(1):163–168. https://doi.org/10.1016/j.bios.2011.11.048
Tran HV, Piro B, Reisberg S, Tran LD, Duc HT, Pham MC (2013) Label-free and reagentless electrochemical detection of micro RNAs using a conducting polymer nanostructured by carbon nanotubes: application to prostate cancer biomarker miR-141. Biosens Bioelectron 49:164–169. https://doi.org/10.1016/j.bios.2013.05.007
Tran HV, Piro B, Reisberg S, Duc HT, Pham MC (2013) Antibodies directed to RNA/DNA hybrids: a novel electrochemical immunosensor for miRNAs detection using graphene-composite electrodes. Anal Chem 85:8469–8474. https://doi.org/10.1021/ac402154z
Kangkamano T, Numnuam A, Limbut W, Kanatharana P, Vilaivan T, Thavarungkul P (2018) Pyrrolidinyl PNA polypyrrole/silver nanofoam electrode as a novel label-free electrochemical miRNA-21 biosensor. Biosens Bioelectron 102:217–225. https://doi.org/10.1016/j.bios.2017.11.024
Wang Y, Zheng D, Tan Q, Wang MX, Gu L-Q (2011) Nanopore-based detection of circulating microRNAs in lung cancer patients. Nat Nanotechnol 6:668–674. https://doi.org/10.1038/nnano.2011.147
Wang Y-H, Huang K-J, Wu X, Ma Y-Y, Song D-L, Du C-Y, Chang S-H (2018) Ultrasensitive supersandwich-type biosensor for enzyme-free amplified microRNA detection based on N-doped graphene/Au nanoparticles and hemin/G-quadruplexes. J Mater Chem B 6:2134–2142. https://doi.org/10.1039/C8TB00061A
Li Y, Yu C, Yang B, Liu Z, Xia P, Wang Q (2018) Target-catalyzed hairpin assembly and metal-organic frameworks mediated nonenzymatic co-reaction for multiple signal amplification detection of miR-122 in human serum. Biosens Bioelectron 105:307–315. https://doi.org/10.1016/j.bios.2017.11.047
Wu X, Chai Y, Yuan R, Su H, Han J (2013) A novel label-free electrochemical microRNA biosensor using Pd nanoparticles as enhancer and linker. Analyst 138:1060–1066. https://doi.org/10.1039/C2AN36506E
Su S, Cao W, Liu W, Lu Z, Zhu D, Chao J, Weng L, Wang L, Fan C, Wang L (2017) Dual-mode electrochemical analysis of microRNA-21 using gold nanoparticle-decorated MoS2 nanosheet. Biosens Bioelectron 94:552–559. https://doi.org/10.1016/j.bios.2017.03.040
Goda T, Masuno K, Nishida J, Kosaka N, Ochiya T, Matsumoto A, Miyahara Y (2012) A label-free electrical detection of exosomal microRNAs using microelectrode array. Chem Commun 48:11942–11944. https://doi.org/10.1039/C2CC36111F
Labib M, Khan N, Ghobadloo SM, Cheng J, Pezacki JP, Berezovski MV (2013) Three-mode electrochemical sensing of ultralow microRNA levels. J Am Chem Soc 135(8):3027–3038. https://doi.org/10.1021/ja308216z
Tran HV, Piro B, Reisberg S, Huy Nguyen L, Dung Nguyen T, Duc HT, Pham MC (2014) An electrochemical ELISA-like immunosensor for miRNAs detection based on screen-printed gold electrodes modified with reduced graphene oxide and carbon nanotubes. Biosens Bioelectron 62:25–30. https://doi.org/10.1016/j.bios.2014.06.014
Salimi A, Kavosi B, Navaee A (2019) Amine-functionalized graphene as an effective electrochemical platform toward easily miRNA hybridization detection. Measurement 143:191–198. https://doi.org/10.1016/j.measurement.2019.05.008
Wan Z, Umer M, Lobino M, Thiel D, Nguyen N-T, Trinchi A, Shiddiky MJA, Gao Y, Li Q (2020) Laser induced self-N-doped porous graphene as an electrochemical biosensor for femtomolar miRNA detection. Carbon 163:385–394. https://doi.org/10.1016/j.carbon.2020.03.043
Wang F, Chu Y, Ai Y, Chen L, Gao F (2019) Graphene oxide with in-situ grown Prussian Blue as an electrochemical probe for microRNA-122. Microchim Acta 186:116. https://doi.org/10.1007/s00604-018-3204-9
Dong H, Jin S, Ju H, Hao K, Xu LP, Lu H, Zhang X (2012) Trace and label-free MicroRNA detection using oligonucleotide encapsulated silver nanoclusters as probes. Anal Chem 84(20):8670–8674. https://doi.org/10.1021/ac301860v
Yin H, Zhou Y, Zhang H, Meng X, Ai S (2012) Electrochemical determination of microRNA-21 based on graphene, LNA integrated molecular beacon, AuNPs and biotin multifunctional bio bar codes and enzymatic assay system. Biosens Bioelectron 33(1):247–253. https://doi.org/10.1016/j.bios.2012.01.014
Li Y, Tian R, Zheng X, Huang R (2016) Amplified electrochemical detection of nucleic acid hybridization via selective preconcentration of unmodified gold nanoparticles. Anal Chim Acta 934:59–65. https://doi.org/10.1016/j.aca.2016.06.035
Fang CS, Kim K, Yu B, Jon S, Kim M-S, Yang H (2017) Ultrasensitive electrochemical detection of miRNA-21 using a zinc finger protein specific to DNA–RNA hybrids. Anal Chem 89(3):2024–2031. https://doi.org/10.1021/acs.analchem.6b04609
Yu LD, Wen YX, Zhang XY, Li NB, Luo HQ (2020) Signal-off photoelectrochemical determination of miRNA-21 using aptamer-modified In2O3@Cu2MoS4 nanocomposite. Microchim Acta 187:561. https://doi.org/10.1007/s00604-020-04540-z
Su S, Hao Q, Yan Z, Dong R, Yang R, Zhu D, Chao J, Zhou Y, Wang L (2019) A molybdenum disulfide@Methylene Blue nanohybrid for electrochemical determination of microRNA-21, dopamine and uric acid. Microchim Acta 186:607. https://doi.org/10.1007/s00604-019-3678-0
Sun Y, Jin H, Jiang X, Gui R (2020) Black phosphorus nanosheets adhering to thionine-doped 2D MOF as a smart aptasensor enabling accurate capture and ratiometric electrochemical detection of target microRNA. Sensors Actuators B Chem 309:127777. https://doi.org/10.1016/j.snb.2020.127777
Schwartz EF, Stollar BD (1969) Antibodies to polyadenylate-polyuridylate copolymers as reagents for double strand RNA and DNA-RNA hybrid complexes. Biochem Biophys Res Commun 35(1):115–120. https://doi.org/10.1016/0006-291x(69)90490-2
Stollar BD (1970) Double-helical polynucleotides: immunochemical recognition of differing conformations. Science 169:609–611. https://doi.org/10.1126/science.169.3945.609
Rudkin GT, Stollar BD (1977) High resolution detection of DNA–RNA hybrids in situ by indirect immunofluorescence. Nature 265:472–473. https://doi.org/10.1038/265472a0
Yoshichika K, Stollar BD (1982) Comparison of poly(A)·poly(dT) and poly(I)·poly(dC) as immunogens for the induction of antibodies to RNA-DNA hybrids. Mol Immunol 19(3):413–420. https://doi.org/10.1016/0161-5890(82)90207-3
Qavi AJ, Kindt JT, Gleeson MA, Bailey RC (2011) Anti-DNA:RNA antibodies and silicon photonic microring resonators: increased sensitivity for multiplexed microRNA detection. Anal Chem 83(15):5949–5956. https://doi.org/10.1021/ac201340s
Hu Z, Zhang A, Storz G, Gottesman S, Leppla SH (2006) An antibody-based microarray assay for small RNA detection. Nucleic Acids Res 37(7):e52. https://doi.org/10.1093/nar/gkl142
Zouari M, Campuzano S, Pingarrón JM, Raouafi N (2018) Amperometric biosensing of miRNA-21 in serum and cancer cells at nanostructured platforms using anti-DNA-RNA hybrid antibodies. ACS Omega 3(8):8923–8931. https://doi.org/10.1021/acsomega.8b00986
Wang M, Yin H, Zhou Y, Sui C, Wang Y, Meng X, Waterhouse GIN, Ai S (2019) Photoelectrochemical biosensor for microRNA detection based on a MoS2/g-C3N4/black TiO2 heterojunction with Histostar@AuNPs for signal amplification. Biosens Bioelectron 128:137–143. https://doi.org/10.1016/j.bios.2018.12.048
Wang M, Li B, Zhou Q, Yin H, Zhou Y, Ai S (2015) Label-free, ultrasensitive and electrochemical immunosensing platform for microRNA detection using anti-DNA:RNA hybrid antibody and enzymatic signal amplification. Electrochim Acta 165:130–113. https://doi.org/10.1016/j.electacta.2015.03.011
Ramnani P, Gao Y, Ozsoz M, Mulchandani A (2013) Electronic detection of microRNA at attomolar level with high specificity. Anal Chem 85(17):8061–8064. https://doi.org/10.1021/ac4018346
Kilic T, Topkaya SN, Ozsoz M (2013) A new insight into electrochemical microRNA detection: a molecular caliper, p19 protein. Biosens Bioelectron 48:165–171. https://doi.org/10.1016/j.bios.2013.04.011
Ahmed N, Foss DV, Powdrill MH, Pezacki JP (2019) Site-specific cross-linking of a p19 viral suppressor of RNA silencing protein and its RNA targets using an expanded genetic code. Biochemistry 58(33):3520–3526. https://doi.org/10.1021/acs.biochem.9b00428
Campuzano S, Pedrero M, Pingarrón JM (2016) Viral protein-based bioanalytical tools for small RNA biosensing. TrAC Trends Anal Chem 79:335–343. https://doi.org/10.1016/j.trac.2015.09.012
Tu W, Cao H, Zhang L, Bao J, Liu X, Dai Z (2016) Dual signal amplification using gold nanoparticles-enhanced zinc selenide nanoflakes and P19 protein for ultrasensitive photoelectrochemical biosensing of microRNA in cell. Anal Chem 88(21):10459–10465. https://doi.org/10.1021/acs.analchem.6b02381
Li C, Liu Z, Cai S, Wen F, Wu D, Liu Y, Wu F, Lan J, Han Z, Chen J (2015) An electrochemical microRNA biosensor based on protein p19 combining an acridone derivate as indicator and DNA concatamers for signal amplification. Electrochem Commun 60:185–189. https://doi.org/10.1016/j.elecom.2015.09.012
Wanunu M, Dadosh T, Ray V, Jin J, McReynolds L, Drndic M (2010) Rapid electronic detection of probe-specific microRNAs using thin nanopore sensors. Nat Nanotechnol 5:807–814. https://doi.org/10.1038/nnano.2010.202
Chen Z, Xie Y, Huang W, Qin C, Yu A, Lai G (2019) Exonuclease-assisted target recycling for ultrasensitive electrochemical detection of microRNA at vertically aligned carbon nanotubes. Nanoscale 11:11262–11269. https://doi.org/10.1039/C9NR02543J
Zhang C, Li D, Li D, Wen K, Yang X, Zhu Y (2019) Rolling circle amplification-mediated in situ synthesis of palladium nanoparticles for the ultrasensitive electrochemical detection of microRNA. Analyst 144:3817–3825. https://doi.org/10.1039/C9AN00427K
Bai Y-Y, Wu Z, Xu C-M, Zhang L, Feng J, Pang D-W, Zhang Z-L (2020) One-to-many single entity electrochemistry biosensing for ultrasensitive detection of microRNA. Anal Chem 91(1):853–858. https://doi.org/10.1021/acs.analchem.9b03492
Wang G, Tian W, Liu X, Ren W, Liu C (2020) New CRISPR-derived microRNA sensing mechanism based on Cas12a self-powered and rolling circle transcription-unleashed real-time crRNA recruiting. Anal Chem 92(9):6702–6708. https://doi.org/10.1021/acs.analchem.0c00680
Kergoat L, Piro B, Berggren M, Pham MC, Yassar A, Horowitz G (2012) DNA detection with a water-gated organic field-effect transistor. Org Electron 13:1–6. https://doi.org/10.1016/j.orgel.2011.09.025
Pallu J, Avci-Adali M, Mackeben P, Mohammadnejad L, Mattana G, Noël V, Piro B (2019) A DNA hydrogel gated organic field effect transistor. Org Electron 75:105402. https://doi.org/10.1016/j.orgel.2019.105402
White SP, Dorfman KD, Frisbie CD (2015) Label-free DNA sensing platform with low-voltage electrolyte-gated transistors. Anal Chem 87(3):1861–1866. https://doi.org/10.1021/ac503914x
Campos R, Borme J, Guerreiro JR, Machado G Jr, Cerqueira MF, Petrovykh DY, Alpuim P (2019) Attomolar label-free detection of DNA hybridization with electrolyte-gated graphene field-effect transistors. ACS Sens 4(2):286–293. https://doi.org/10.1021/acssensors.8b00344
Matsuura S, Ono H, Kawasaki S, Kuang Y, Fujita Y, Saito H (2018) Synthetic RNA-based logic computation in mammalian cells. Nat Commun 9:4847. https://doi.org/10.1038/s41467-018-07181-2
Zhao X-P, Liu F-F, Hu W-C, Younis MR, Wang C, Xia X-H (2019) Biomimetic nanochannel-ionchannel hybrid for ultrasensitive and label-free detection of microRNA in cells. Anal Chem 91(5):3582–3589. https://doi.org/10.1021/acs.analchem.8b05536
Bruch R, Baaske J, Chatelle C, Meirich M, Madlener S, Weber W, Dincer C, Urban GA (2019) CRISPR/Cas13a-powered electrochemical microfluidic biosensor for nucleic acid amplification-free miRNA diagnostics. Adv Mater 31(51):1905311. https://doi.org/10.1002/adma.201905311
Pingarron JM, Campuzano S, Ruiz-Valdepenas-Montiel V, Torrente-Rodriguez RM, Montoya JJ (2017) Magnetic beads-based electrochemical biosensor. Patent n°WO2017137192A1
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tran, H.V., Piro, B. Recent trends in application of nanomaterials for the development of electrochemical microRNA biosensors. Microchim Acta 188, 128 (2021). https://doi.org/10.1007/s00604-021-04784-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04784-3