Abstract
Core-shell structured magnetic covalent organic framework (Fe3O4@COF) nanospheres were rapidly synthesized at room temperature using the monodisperse Fe3O4 nanoparticles (NPs) as magnetic core and benzene-1,3,5-tricarbaldehyde (BTA) and 3,3′-dihydroxybenzidine (DHBD) as two building blocks (denoted as Fe3O4@BTA-DHBD), respectively. They can serve as a mass spectrometry probe for rapid and high-throughput screening of bisphenols (BPs) from pharmaceuticals and personal care products (PPCPs) by surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF-MS). The Fe3O4@BTA-DHBD nanospheres showed some superior features involving average pore size distribution (2.82 nm), high magnetization values (42.5 emu g−1), high specific surface area (82.96 m2 g−1), and good chemical/thermal stability. It was used as both ideal adsorbent for enrichment of BPs and new substrate to assist ionization in SELDI-TOF-MS. The method exhibited good linearity in the range 0.05–4000 ng mL−1 with correlation coefficients (r) higher than 0.9920. Low limits of detection (LODs) (500 pg mL−1 for bisphenol A (BPA), 2 pg mL−1 for bisphenol B (BPB), 28 pg mL−1 for bisphenol C (BPC), 60 pg mL−1 for bisphenol F (BPF), 33 pg mL−1 for bisphenol AF (BPAF), 200 pg mL−1 for bisphenol BP (BPBP), 10 pg mL−1 for bisphenol S (BPS), 90 pg mL−1 for tetrabromobisphenol A (BPA(Br)4), and 380 pg mL−1 for tetrabromobisphenol S (BPS(Br)4)) and good recoveries (80.6–115%) of BPs in PPCPs were achieved. The relative standard deviations (RSDs) of spot-to-spot (n = 10) and sample-to-sample (n = 5) were in the ranges 5–11% and 5–12%, respectively. The dual-function platform was successfully applied to the quantitative determination of BPs in PPCPs. It not only expanded the scope of the application of COFs but also provided an alternative strategy for the determination of hazardous compounds in PPCPs.

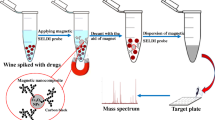
Schematic representation of the synthesis of core-shell structured magnetic covalent organic framework nanospheres (Fe3O4@COFs) and its application in the analysis of bisphenols by using Fe3O4@BTA-DHBD nanospheres as a MS probe based on surface-enhanced laser desorption/ionization time-of-flight mass spectrometry.






Similar content being viewed by others
References
Ebele AJ, Abou-Elwafa Abdallah M, Harrad S (2017) Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg Contam 3:1–16. https://doi.org/10.1016/j.emcon.2016.12.004
Awfa D, Ateia M, Fujii M, Johnson MS, Yoshimura C (2018) Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO2 composites: a critical review of recent literature. Water Res 142:26–45. https://doi.org/10.1016/j.watres.2018.05.036
Liu JL, Wong MH (2013) Pharmaceuticals and personal care products (PPCPs): a review on environmental contamination in China. Environ Int 59:208–224. https://doi.org/10.1016/j.envint.2013.06.012
Selvaraj KK, Shanmugam G, Sampath S, Larsson DG, Ramaswamy BR (2014) GC-MS determination of bisphenol A and alkylphenol ethoxylates in river water from India and their ecotoxicological risk assessment. Ecotoxicol Environ Saf 99:13–20. https://doi.org/10.1016/j.ecoenv.2013.09.006
Eladak S, Grisin T, Moison D, Guerquin MJ, N’Tumba-Byn T, Pozzi-Gaudin S, Benachi A, Livera G, Rouiller-Fabre V, Habert R (2015) A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil Steril 103:11–21. https://doi.org/10.1016/j.fertnstert.2014.11.005
Gong SX, Wang XL, Liu W, Wang ML, Wang X, Wang ZW, Zhao RS (2017) Aminosilanized magnetic carbon microspheres for the magnetic solid-phase extraction of bisphenol A, bisphenol AF, and tetrabromobisphenol A from environmental water samples. J Sep Sci 40:1755–1764
Chen L, He YT, Lei ZX, Gao CL, Xie Q, Tong P, Lin ZA (2018) Preparation of core-shell structured magnetic covalent organic framework nanospheres for magnetic solid-phase extraction of bisphenols from human serum sample. Talanta 181:296–304. https://doi.org/10.1016/j.talanta.2018.01.036
Baghayeri M, Ansari R, Nodehi M, Razavipanah I, Veisi H (2018) Voltammetric aptasensor for bisphenol A based on the use of a MWCNT/Fe3O4@gold nanocomposite. Microchim Acta 185:320. https://doi.org/10.1007/s00604-018-2838-y
Deng ZH, Wang X, Wang XL, Gao CL, Dong L, Wang ML, Zhao RS (2019) A core-shell structured magnetic covalent organic framework (type Fe3O4@ COF) as a sorbent for solid-phase extraction of endocrine-disrupting phenols prior to their quantitation by HPLC. Microchim Acta 186:108. https://doi.org/10.1007/s00604-018-3198-3
Cunha SC, Fernandes JO (2013) Assessment of bisphenol A and bisphenol B in canned vegetables and fruits by gas chromatography–mass spectrometry after QuEChERS and dispersive liquid–liquid microextraction. Food Control 33:549–555. https://doi.org/10.1016/j.foodcont.2013.03.028
Regueiro J, Wenzl T (2015) Development and validation of a stable-isotope dilution liquid chromatography-tandem mass spectrometry method for the determination of bisphenols in ready-made meals. J Chromatogr A 1414:110–121. https://doi.org/10.1016/j.chroma.2015.08.037
Cheng Y, Nie XM, Wu HQ, Hong YH, Yang BC, Liu T, Zhao D, Wang JF, Yao GH, Zhang F (2017) A high-throughput screening method of bisphenols, bisphenols digycidyl ethers and their derivatives in dairy products by ultra-high performance liquid chromatography-tandem mass spectrometry. Anal Chim Acta 950:98–107. https://doi.org/10.1016/j.aca.2016.11.006
Bonfoh SI, Li D, Xiong X, Du ZF, Xiong CM, Jiang HL (2020) Novel PEP-PAN@PSF rods extraction of EDCs in environmental water, sediment, and fish homogenate followed by pre-column derivatization and UHPLC-MS/MS detection. Talanta 210:120661. https://doi.org/10.1016/j.talanta.2019.120661
Chen YS, Ding J, He XM, Xu J, Feng YQ (2018) Synthesis of tellurium nanosheet for use in matrix assisted laser desorption/ionization time-of-flight mass spectrometry of small molecules. Microchim Acta 185:368. https://doi.org/10.1007/s00604-018-2882-7
Hu K, Lv YX, Ye FG, Chen T, Zhao SL (2019) Boric-acid-functionalized covalent organic framework for specific enrichment and direct detection of cis-Diol-containing compounds by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal Chem 91:6353–6362. https://doi.org/10.1021/acs.analchem.9b01376
Lin ZA, Zheng JN, Lin G, Tang Z, Yang XQ, Cai ZW (2015) Negative ion laser desorption/ionization time-of-flight mass spectrometric analysis of small molecules using graphitic carbon nitride nanosheet matrix. Anal Chem 87:8005–8012. https://doi.org/10.1021/acs.analchem.5b02066
Wang J, Liu Q, Gao Y, Wang YW, Guo LQ, Jiang GB (2015) High-throughput and rapid screening of low-mass hazardous compounds in complex samples. Anal Chem 87:6931–6936. https://doi.org/10.1021/acs.analchem.5b01550
Lin ZA, Bian W, Zheng JN, Cai ZW (2015) Magnetic metal-organic framework nanospheres for enrichment and direct detection of small molecules by negative-ion matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Chem Commun 51:8785–8788. https://doi.org/10.1039/c5cc02495a
Zhu Q, Teng F, Wang ZS, Wang YL, Lu N (2019) Superhydrophobic glass substrates coated with fluorosilane-coated silica nanoparticles and silver nanoparticles for surface-assisted laser desorption/ionization mass spectrometry. ACS Appl Nano Mater 2:3813–3818. https://doi.org/10.1021/acsanm.9b00688
Huang X, Liu Q, Jiang GB (2019) Tuning the performance of graphene as a dual-ion-mode MALDI matrix by chemical functionalization and sample incubation. Talanta 199:532–540. https://doi.org/10.1016/j.talanta.2019.03.010
Wang S, Niu HY, Cao D, Cai YQ (2019) Covalent-organic frameworks as adsorbent and matrix of SALDI-TOF MS for the enrichment and rapid determination of fluorochemicals. Talanta 194:522–527. https://doi.org/10.1016/j.talanta.2018.10.071
Niu HY, Wang SH, Tan YX, Song XW, Cai YQ (2016) Simultaneous and direct analysis of multiple types of organic contaminants in water based on a MOF decorated with a suitable quantity of Au nanoparticles, using SALDI-TOF MS. RSC Adv 6:99919–99923. https://doi.org/10.1039/c6ra19635g
Huang X, Liu Q, Fu JJ, Nie Z, Gao K, Jiang GB (2016) Screening of toxic chemicals in a single drop of human whole blood using ordered mesoporous carbon as a mass spectrometry probe. Anal Chem 88:4107–4113. https://doi.org/10.1021/acs.analchem.6b00444
Zeng YF, Zou RY, Luo Z, Zhang HC, Yao X, Ma X, Zou RQ, Zhao YL (2015) Covalent organic frameworks formed with two types of covalent bonds based on orthogonal reactions. J Am Chem Soc 137:1020–1023. https://doi.org/10.1021/ja510926w
Huang N, Zhai LP, Coupry DE, Addicoat MA, Okushita K, Nishimura K, Heine T, Jiang DL (2016) Multiple-component covalent organic frameworks. Nat Chem 7:12325. https://doi.org/10.1038/ncomms12325
Lin CY, Zhang D, Zhao ZH, Xia ZH (2018) Covalent organic framework electrocatalysts for clean energy conversion. Adv Mater 30:1703646. https://doi.org/10.1002/adma.201703646
Ding SY, Wang W (2013) Covalent organic frameworks (COFs): from design to applications. Chem Soc Rev 42:548–568. https://doi.org/10.1039/c2cs35072f
Baldwin LA, Crowe JW, Pyles DA, McGrier PL (2016) Metalation of a mesoporous three-dimensional covalent organic framework. J Am Chem Soc 138:15134–15137. https://doi.org/10.1021/jacs.6b10316
Segura JL, Mancheno MJ, Zamora F (2016) Covalent organic frameworks based on Schiff-base chemistry: synthesis, properties and potential applications. Chem Soc Rev 45:5635–5671. https://doi.org/10.1039/c5cs00878f
Xu H, Gao J, Jiang DL (2015) Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat Chem 7:905–912. https://doi.org/10.1038/nchem.2352
Bunck DN, Dichtel WR (2012) Internal functionalization of three-dimensional covalent organic frameworks. Angew Chem Int Ed 51:1885–1889. https://doi.org/10.1002/anie.201108462
Lohse MS, Bein T (2018) Covalent organic frameworks: structures, synthesis, and applications. Adv Funct Mater 28:1705553. https://doi.org/10.1002/adfm.201705553
Ge JL, Xiao JD, Liu LL, Qiu LG, Jiang X (2016) Facile microwave-assisted production of Fe3O4 decorated porous melamine-based covalent organic framework for highly selective removal of Hg2+. J Porous Mater 23:791–800. https://doi.org/10.1007/s10934-016-0134-y
Mellah A, Fernandes SPS, Rodríguez R, Otero J, Paz J, Cruces J, Medina DD, Djamila H, Espiña B, Salonen LM (2018) Adsorption of pharmaceutical pollutants from water using covalent organic frameworks. Chem Eur J 24:10601–10605. https://doi.org/10.1002/chem.201801649
Qian HL, Yang CX, Wang WL, Yang C, Yan XP (2018) Advances in covalent organic frameworks in separation science. J Chromatogr A 1542:1–18. https://doi.org/10.1016/j.chroma.2018.02.023
Song YH, Ma RY, Hao L, Yang XM, Wang C, Wu QH, Wang Z (2018) Application of covalent organic framework as the adsorbent for solid-phase extraction of trace levels of pesticide residues prior to high-performance liquid chromatography-ultraviolet detection. J Chromatogr A 1572:20–26. https://doi.org/10.1007/s00604-017-2408-8
Wang MT, Zhou X, Zang XH, Pang YC, Chang QY, Wang C, Wang Z (2018) Determination of pesticides residues in vegetable and fruit samples by solid-phase microextraction with a covalent organic framework as the fiber coating coupled with gas chromatography and electron capture detection. J Sep Sci 41:4038–4046. https://doi.org/10.1002/jssc.201800644
Zhao WJ, Wang XY, Guo JH, Guo Y, Lan C, Xie FW, Zong SY, He LJ, Zhang SS (1681) Evaluation of sulfonic acid functionalized covalent triazine framework as a hydrophilic-lipophilic balance/cation-exchange mixed-mode sorbent for extraction of benzimidazole fungicides in vegetables, fruits and juices. J Chromatogr A 2020:460847. https://doi.org/10.1016/j.chroma.2019.460847
Zhang YH, Song YY, Wu J, Li RJ, Hu D, Lin ZA, Cai ZW (2019) A magnetic covalent organic framework as an adsorbent and a new matrix for enrichment and rapid determination of PAHs and their derivatives in PM2.5 by surface-assisted laser desorption/ionization-time of flight-mass spectrometry. Chem Commun 55:3745–3748. https://doi.org/10.1039/x0xx00000x
Zhang ZZ, Zhang J, Wang Y, Tong Y, Zhang L (2017) Controlled synthesis of hollow porous carbon spheres for enrichment and simultaneous determination of nine bisphenols from real samples. Talanta 167:428–435. https://doi.org/10.1016/j.talanta.2017.02.037
Wang XY, Deng CH (2015) Preparation of magnetic graphene@polydopamine @Zr-MOF material for the extraction and analysis of bisphenols in water samples. Talanta 144:1329–1335. https://doi.org/10.1016/j.talanta.2015.08.014
Wang LL, Zhang ZZ, Zhang J, Zhang L (2016) Magnetic solid-phase extraction using nanoporous three dimensional graphene hybrid materials for high-capacity enrichment and simultaneous detection of nine bisphenol analogs from water sample. J Chromatogr A 1463:1–10. https://doi.org/10.1016/j.chroma.2016.08.003
Funding
This work was financially supported from the National Natural Science Foundation of China (91843301, 21974021 and 21675025) and the Natural Science Foundation of Fujian Province (2018J01683).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1.40 mb)
Rights and permissions
About this article
Cite this article
Sun, Q., Gao, C., Ma, W. et al. High-throughput screening of bisphenols using magnetic covalent organic frameworks as a SELDI-TOF-MS probe. Microchim Acta 187, 370 (2020). https://doi.org/10.1007/s00604-020-04340-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04340-5