Abstract
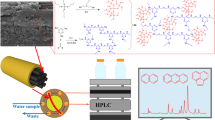
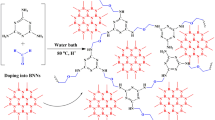
A combination between an ionic liquid and melamine-formaldehyde aerogel on the carbon fibers was developed for in-tube solid-phase microextraction of estrogens with high efficiency. The sorbent has a high enrichment capability for several estrogens. Scanning electron microscopy showed that the aerogel on the carbon fibers has a porous three-dimensional network structure. Several important parameters such as sampling volume, sampling rate, the concentration of organic solvent in sample, pH value of sample as well as desorption time were optimized towards estrogen targets. Comparing with melamine-formaldehyde aerogel coating, the coating gave higher extraction efficiency. Comparing with melamine-formaldehyde aerogel coating, the new coating displays higher extraction efficiency. An online analytical method of estrogens was established, by the combination between in-tube solid-phase microextraction and high performance liquid chromatography with diode array detector. Analytical figures of merit include low limits of detection (<0.20 μg L−1), wide linearity (0.15–20 μg L−1), high enrichment factors (1028–1256), good extraction repeatability (RSDs<2.5%) and satisfactory preparation repeatability (RSDs<10.5%). The method was applied to the determination of trace estrogen targets in plastic bottle, tap water and surface water.

Schematic representation of online combination between in-tube solid-phase microextraction and high performance liquid chromatography, based on an ionic liquid (IL)-modified melamine-formaldehyde (MF) aerogel coating on carbon fibers (CFs) in a polyether-etherketone (PEEK) tube.



Similar content being viewed by others
References
Moon I, Yoon S, Chun K, Oh J (2015) Highly elastic and conductive N-doped monolithic graphene aerogels for multifunctional applications. Adv Funct Mater 25:6976–6984
Yoldas E (1975) Alumina sol preparation from alkoxides. Am Ceram Soc Bull 54:289–290
Baktash M, Bagheri H (2017) Silica aerogel coated on metallic wire by phase separation of polystyrene for in-tube solid phase microextraction. J Chromatogr A 1500:69–75
Wang X, Lu M, Wang H, Huang P, Ma X, Cao C, Du X (2016) Three-dimensional graphene aerogel-mesoporous carbon composites as novel coatings for solid-phase microextraction for the efficient enrichment of brominated flame retardants. New J Chem 40:6308–6314
Berestok T, Guardia P, Du R, Javier B, Colombo M, Esteade S, Peiro F, Brock S, Cabot A (2018) Metal oxide aerogels with controlled crystallinity and faceting from the epoxide-driven cross-linking of colloidal nanocrystals. ACS Appl Mater Interfaces 10:16041–16048
Alviso C, Pekala R (1992) Melamine-formaldehyde aerogels. Polym Preprints 32:242–243
Al-Muhtaseb S, Ritter J (2003) Preparation and properties of resorcinol-formaldehyde organic and carbon gels. Adv Mater 15:101–114
Zhang M, Cheng J, Xuan X (2017) Pt/graphene aerogel deposited in Cu foam as a 3D binder-free cathode for CO2 reduction into liquid chemicals in a TiO2 photoanode-driven photoelectrochemical cell. Chem Eng J 322:22–32
Wang X, Lu M, Wang H, Pei Y, Rao H, Du X (2015) Three-dimensional graphene aerogels-mesoporous silica frameworks for superior adsorption capability of phenols. Sep Purif Technol 153:7–13
Chen M, Li Z, Geng Y, Zhao H, He S, Li Q, Zhang L (2018) Adsorption behavior of thorium on N,N,N',N'-tqetraoctyldiglycolamide (TODGA) impregnated graphene aerogel. Talanta 181:311–317
Roostaie A, Mohammadiazar S, Bargozin H, Ehteshami S (2018) A modified nanoporous silica aerogel as a new sorbent for needle trap extraction of chlorobenzenes from water samples. Chromatographia 81:649–655
Joul P, Vaher M, Kuhtinskaja M (2018) Evaluation of carbon aerogel-based solid-phase extraction sorbent for the analysis of sulfur mustard degradation products in environmental water samples. Chemosphere 198:460–468
He S, Bi Y, Zhang Y, Cao H, Shi X, Luo X, Zhang L (2015) One-pot synthesis and characterization of acid-catalyzed melamine formaldehyde/SiO2 aerogel via sol-gel technology. J Sol-Gel Sci Technol 74:175–180
Zhang Y, Zhu J, Ren H, Bi Y, Zhang L (2017) Synthesis and properties of melamine-starch hybrid aerogels cross-linked with formaldehyde. J Sol-Gel Sci Technol 83:44–52
Welton T (1999) Room-temperature ionic liquids: solvents for synthesis and catalysis. Chem Rev 99:2071–2084
Huang K, Chen Y, Zhang X, Xia S, Wu Y, Hu X (2014) SO2 absorption in acid salt ionic liquids/sulfolane binary mixtures: experimental study and thermodynamic analysis. Chem Eng J 237:478–486
Harada M, Kawasaki C, Saijo K, Demizu M, Kimura Y (2010) Photochemical synthesis of silver particles using water-in-ionic liquid microemulsions in high-pressure CO2. J Colloid Interface Sci 343:537–545
Feng J, Mao H, Wang X, Tian Y, Luo C, Sun M (2018) Ionic liquid chemically bonded basalt fibers for in-tube solid-phase microextraction. J Sep Sci 41:1149–1155
Feng J, Wang X, Tian Y, Luo C, Sun M (2017) Poly(ionic liquids)-coated stainless-steel wires packed into a polyether ether ketone tube for in-tube solid-phase microextraction. J Sep Sci 40:4773–4779
Sun M, Feng J, Bu Y, Luo C (2016) Ionic liquid coated copper wires and tubes for fiber-in-tube solid-phase microextraction. J Chromatogr A 1458:1–8
Xiao R, Zhang X, Zhang X, Niu J, Lu M, Liu X, Cai Z (2017) Analysis of flavors and fragrances by HPLC with Fe3O4@GO magnetic nanocomposite as the adsorbent. Talanta 166:262–267
Zeng J, Chen J, Li M, Subhan F, Chong F, Wen C, Yu J, Cui B, Chen X (2015) Determination of amphetamines in biological samples using electro enhanced solid-phase microextraction-gas chromatography. J Chromatogr B 1000:169–175
Liu L, Meng W, Zhou Y, Wang X, Xu G, Wang M, Lin J, Zhao R (2019) β-ketoenamine-linked covalent organic framework coating for ultra-high-performance solid-phase microextraction of polybrominated diphenyl ethers from environmental samples. Chem Eng J 356:926–933
Zhang J, Li W, Zhu W, Yang Y, Qin P, Zhou Q, Lu M, Cai Z (2019) Mesoporous graphitic carbon nitride as an efficient sorbent for extraction of sulfonamides prior to HPLC analysis. Microchim Acta 186(279). https://doi.org/10.1007/s00604-019-3394-9
Liu L, Meng W-K, Li L, Xu G-J, Wang X, Chen L-Z, Wang M-L, Lin J-M, Zhao R-S (2019) Facile room-temperature synthesis of a spherical mesoporous covalent organic framework for ultrasensitive solid-phase microextraction of phenols. Chem Eng J 369:920–927
Wen C, Li M, Li W, Li Z, Duan W, Li Y, Zhou J, Li X, Zeng J (2017) Graphene deposited onto aligned zinc oxide nanorods as an efficient coating for headspace solid-phase microextraction of gasoline fractions from oil samples. J Chromatogr A 1530:45–50
Liu X, Jia Y, Zhang H, Liu M (2008) Highly sensitive analysis of substituted aniline compounds in water samples by using oxidized multiwalled carbon nanotubes as an in-tube solid-phase microextraction medium. J Chromatogr A 1212:10–15
Bu Y, Feng J, Tian Y, Wang X, Sun M, Luo C (2017) An organically modified silica aerogel for online in-tube solid-phase microextraction. J Chromatogr A 1517:203–208
Ling X, Zhang W, Chen Z (2016) Electrochemically modified carbon fiber bundles as selective sorbent for online solid-phase microextraction of sulfonamides. Microchim Acta 183:813–820
Saito Y, Kawazoe M, Hayashida M, Jinno K (2000) Direct coupling of microcolumn liquid chromatography with in-tube solid-phase microextraction for the analysis of antidepressant drugs. Analyst 125:807–809
Penalver A, Pocurull E, Borrull F, Marce R (2002) Method based on solid-phase microextraction-high-performance liquid chromatography with UV and electrochemical detection to determine estrogenic compounds in water samples. J Chromatogr A 964:153–160
Wang C, Yang L, Li N, Zhang X, Guo Y, Li C (2017) Development of immunoaffinity solid phase microextraction rods for analysis of three estrogens in environmental water samples. J Chromatogr B 1061:41–48
Zou Y, Li Y, Jin H, Tang H, Zou D, Liu M, Yang Y (2012) Determination of estrogens in human urine by high-performance liquid chromatography/diode array detection with ultrasound-assisted cloud-point extraction. Anal Biochem 421:378–384
Hu C, He M, Chen B, Zhong C, Hu B (2013) Polydimethylsiloxane/metal-organic frameworks coated stir bar sorptive extraction coupled to high performance liquid chromatography-ultraviolet detector for the determination of estrogens in environmental water samples. J Chromatogr A 1310:21–30
Chen L, Mei M, Huang X, Yuan D (2016) Sensitive determination of estrogens in environmental waters treated with polymeric ionic liquid-based stir cake sorptive extraction and liquid chromatographic analysis. Talanta 152:98–104
Acknowledgements
This research work was financially supported by the National Natural Science Foundation of China (NSFC, No. 21777054) and the Shandong Provincial Natural Science Foundation of China (Nos. ZR2017MB043 and ZR2019MB058).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 680 kb)
Rights and permissions
About this article
Cite this article
Feng, J., Wang, X., Han, S. et al. An ionic-liquid-modified melamine-formaldehyde aerogel for in-tube solid-phase microextraction of estrogens followed by high performance liquid chromatography with diode array detection. Microchim Acta 186, 769 (2019). https://doi.org/10.1007/s00604-019-3909-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3909-4