Abstract
The first example of metallic bismuth encapsulated into a mesoporous metal-organic framework of the type MIL-101(Cr) matrix is presented. Bi(III)-impregnated MIL-101(Cr) (Bi(III)/MIL-101(Cr)) was dropped onto a conductive carbon cloth electrode (CCE). Then, bismuth was generated by electrochemical reduction of the Bi(III)/MIL-101(Cr) supported on CCE (Bi/MIL-101(Cr)/CCE). The resulting Bi/MIL-101(Cr)/CCE display impressive performance in terms of peak currents for the ions Cd(II) and Pb(II) when compared to the single-component counterparts. Differential pulse anodic stripping voltammetry (DPASV) enabled sensing of the two ions over linear working range of 0.1 to 30 μg L−1 and 30 to 90 μg L−1. The parameters are refined before the detection of two metal ions, including the amount of bismuth in MIL-101(Cr), optimum pH (5.0), deposition potential (−1.2 V) and deposition time (600 s). The respective detection limits are 60 and 70 ng L−1 (at S/N = 3). This is strikingly lower than the guideline values of domestic water given by the WHO which are 3 μg L−1 for Cd(II) and 10 μg L−1 for Pb(II). The Bi/MIL-101(Cr) onto CCE is fairly specific for Cd(II) (at around −0.76 V) and Pb(II) (at around −0.54 V), well reproducible and has excellent recovery in real water analysis.

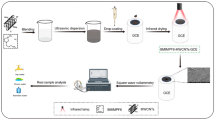
Schematic illustration of the preparation of a Bi(III)/MIL-101(Cr) metal-organic framework, its deposition on a carbon cloth electrode (CCE), and its application for detection of Cd(II) and Pb(II) by differential pulse adsorptive stripping voltammetry (DPASV).










Similar content being viewed by others
References
Li H, Eddaoudi M, O'Keeffe M, Yaghi OM (1999) Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 402:276–279
Panella B, Hirscher M (2005) Hydrogen physisorption in metal-organic porous crystals. Adv Mater 17:538–541
Chughtai AH, Ahmad N, Younus HA, Laypkov A, Verpoort F (2015) Metal-organic frameworks: versatile heterogeneous catalysts for efficient catalytic organic transformations. Chem Soc Rev 44:6804–6849
Stavila V, Talin AA, Allendorf MD (2014) MOF-based electronic and opto-electronic devices. Chem Soc Rev 43:5994–6010
Kumar P, Deep A, Kim KH (2015) Metal organic frameworks for sensing applications. TrAC Trends Anal Chem 73:39–53
Zhou J, Li X, Yang L, Yan S, Wang M, Cheng D, Chen Q, Dong Y, Liu P, Cai W, Zhang C (2015) The Cu-MOF-199/single-walled carbon nanotubes modified electrode for simultaneous determination of hydroquinone and catechol with extended linear ranges and lower detection limits. Anal Chim Acta 899:57–65
Lantao L, Yanli Z, Shuang L, Maotian X (2018) The applications of metal−organic frameworks in electrochemical sensors. ChemElectroChem 5:6–19
Shi E, Lin H, Wang Q, Zhang F, Shi S, Zhang T, Li X, Niu H, Qu F (2017) Synergistic effect of the composite films formed by zeolitic imidazolate framework 8 (ZIF-8) and porous nickel films for enhanced amperometric sensing of hydrazine. Dalton Trans 46:554–563
Anik Ü, Timur S, Dursun Z (2019) Metal organic frameworks in electrochemical and optical sensing platforms: a review. Microchim Acta 186:196
Fei-Yan Y, Rui Z, Hailong W, Li-Feng C, Lei H, Hai-Long J, Qiang X (2017) Metal–organic frameworks and their composites: synthesis and electrochemical applications. Small Methods 1:1700187
Zhang L, Liu H, Shi W, Cheng P (2019) Synthesis strategies and potential applications of metal-organic frameworks for electrode materials for rechargeable lithium ion batteries. Coord Chem Rev 388:293–309
Moon HR, Lim D-W, Suh MP (2013) Fabrication of metal nanoparticles in metal–organic frameworks. Chem Soc Rev 42:1807–1824
Shi L, Zhu X, Liu T, Zhao H, Lan M (2016) Encapsulating cu nanoparticles into metal-organic frameworks for nonenzymatic glucose sensing. Sensors Actuators B Chem 227:583–590
Samadi-Maybodi A, Ghasemi S, Ghaffari-Rad H (2015) Ag-doped zeolitic imidazolate framework-8 nanoparticles modified CPE for efficient electrocatalytic reduction of H2O2. Electrochim Acta 163:280–287
Hosseini H, Ahmar H, Dehghani A, Bagheri A, Fakhari AR, Amini MM (2013) Au-SH-SiO2 nanoparticles supported on metal-organic framework (Au-SH-SiO2@Cu-MOF) as a sensor for electrocatalytic oxidation and determination of hydrazine. Electrochim Acta 88:301–309
Yuan B, Zhang J, Zhang R, Shi H, Wang N, Li J, Ma F, Zhang D (2016) Cu-based metal–organic framework as a novel sensing platform for the enhanced electro-oxidation of nitrite. Sensors Actuators B Chem 222:632–637
Song Y, Xu M, Gong C, Shen Y, Wang L, Xie Y, Wang L (2018) Ratiometric electrochemical glucose biosensor based on GOD/AuNPs/Cu-BTC MOFs/macroporous carbon integrated electrode. Sensors Actuators B Chem 257:792–799
Roushani M, Valipour A, Saedi Z (2016) Electroanalytical sensing of Cd2+ based on metal–organic framework modified carbon paste electrode. Sensors Actuators B Chem 233:419–425
Wang X, Yang C, Zhu S, Yan M, Ge S, Yu J (2017) 3D origami electrochemical device for sensitive Pb2+ testing based on DNA functionalized iron-porphyrinic metal-organic framework. Biosens Bioelectron 87:108–115
Cobbina SJ, Chen Y, Zhou Z, Wu X, Zhao T, Zhang Z, Feng W, Wang W, Li Q, Wu X, Yang L (2015) Toxicity assessment due to sub-chronic exposure to individual and mixtures of four toxic heavy metals. J Hazard Mater 294:109–120
Gumpu MB, Sethuraman S, Krishnan UM, Rayappan JBB (2015) A review on detection of heavy metal ions in water – an electrochemical approach. Sensors Actuators B Chem 213:515–533
Cui L, Wu J, Ju H (2015) Electrochemical sensing of heavy metal ions with inorganic, organic and bio-materials. Biosens Bioelectron 63:276–286
Chen C, Niu X, Chai Y, Zhao H, Lan M (2013) Bismuth-based porous screen-printed carbon electrode with enhanced sensitivity for trace heavy metal detection by stripping voltammetry. Sensors Actuators B Chem 178:339–342
Aragay G, Merkoçi A (2012) Nanomaterials application in electrochemical detection of heavy metals. Electrochim Acta 84:49–61
Wang J, Lu J, Hocevar SB, Farias PAM, Ogorevc B (2000) Bismuth-coated carbon electrodes for anodic stripping voltammetry. Anal Chem 72:3218–3222
Moor C, Lymberopoulou T, Dietrich VJ (2001) Determination of heavy metals in soils, sediments and geological materials by ICP-AES and ICP-MS. Microchim Acta 136:123–128
Achterberg EP, Braungardt C (1999) Stripping voltammetry for the determination of trace metal speciation and in-situ measurements of trace metal distributions in marine waters. Anal Chim Acta 400:381–397
Taillefert M, Luther GW, Nuzzio DB (2000) The application of electrochemical tools for in situ measurements in aquatic systems. Electroanalysis 12:401–412
Férey G, Mellot-Draznieks C, Serre C, Millange F, Dutour J, Surblé S, Margiolaki I (2005) A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 309:2040–2042
Yang C-X, Chen Y-J, Wang H-F, Yan XP (2011) High-performance separation of fullerenes on metal–organic framework MIL-101(Cr). Chem Eur J 17:11734–11737
Wang Q, Shao L, Ma Z, Xu J, Li Y, Wang C (2018) Hierarchical porous PANI/MIL-101 nanocomposites based solid-state flexible supercapacitor. Electrochim Acta 281:582–593
Khan NA, Kang IJ, Seok HY, Jhung SH (2011) Facile synthesis of nano-sized metal-organic frameworks, chromium-benzenedicarboxylate, MIL-101. Chem Eng J 166:1152–1157
Gérard F, Christian S, Caroline MD, Franck M, Suzy S, Julien D, Irène M (2004) A hybrid solid with giant pores prepared by a combination of targeted chemistry, simulation, and powder diffraction. Angew Chem 116:6456–6461
Abdullah AH, Abdullah EA, Zainal Z, Hussein MZ, Ban TK (2012) Adsorptive performance of penta-bismuth hepta-oxide nitrate, Bi5O7NO3, for removal of methyl orange dye. Water Sci Technol 65:1632–1638
Yang F, Yang CX, Yan XP (2015) Post-synthetic modification of MIL-101(Cr) with pyridine for high-performance liquid chromatographic separation of tocopherols. Talanta 137:136–142
Li S, Yang Y, Liu L, Zhao Q (2018) Electron transfer-induced catalytic enhancement over bismuth nanoparticles supported by N-doped graphene. Chem Eng J 334:1691–1698
Duan S, Huang Y (2017) Electrochemical sensor using NH2-MIL-88(Fe)-rGO composite for trace Cd2+, Pb2+, and Cu2+ detection. J Electroanal Chem 807:253–260
Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (Nos. 21771047, 21403048, 21401147 and 21571045), University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (No. UNPYSCT-2017183), Harbin Science and Technology Bureau (2016RAQXJ161), and PhD Research Startup Program of Harbin Normal University, China (No. XKB201310).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 1.68 mb)
Rights and permissions
About this article
Cite this article
Shi, E., Yu, G., Lin, H. et al. The incorporation of bismuth(III) into metal-organic frameworks for electrochemical detection of trace cadmium(II) and lead(II). Microchim Acta 186, 451 (2019). https://doi.org/10.1007/s00604-019-3522-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3522-6