Abstract
Carbon dots co-doped with nitrogen and sulfur (NSCDs) were obtained from thiourea and TAE (Tris-acetate-ethylenediamine) buffer using microwave assisted hydrothermal synthesis. The synergistic presence of nitrogen and sulfur as a dopant results in teasing fluorescence properties and a fluorescence quantum yield of 57%. An HR-TEM study showed the NSCDs to be mono-dispersed and seemingly spherical with an average hydrodynamic diameter of 3.6 ± 0.88 nm. The NSCDs are nontoxic as proven by an MTT assay for cytotoxicity. The optical characterization was done by using UV-Vis absorption and fluorescence spectroscopy which revealed excitation wavelength-dependent multicolor emissions. The characterization of surface topology was done by using X-ray diffraction, FTIR, and X-ray photoelectron spectroscopy. The NSCDs were used to image various pathogenic bacteria (E. coli, Klebsiella, Pseudomonas & Staphylococcus) and human buccal epithelial cells by applying multicolor fluorometry.

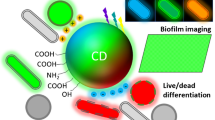
Schematic presentation of microwave-assisted hydrothermal synthesis of nitrogen and sulfur doped carbon dots (NSCD) based on Thiourea and 50X Tris-acetate-ethylenediamine (TAE) buffer having multicolor fluorescence, used for tagging and imaging pathogenic bacteria and Human buccal epithelial cells using fluorescence microscope.








Similar content being viewed by others
Change history
24 August 2019
The published version of this article, unfortunately, contains error. Figure 2 image was available in the multiple submissions during reviewing of the manuscript. But during the final submission, the author was asked to provide the word document of the manuscript with good resolution of the images.
References
Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK (2018) Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol 9:1050–1074
DaCosta MV, Doughan S, Han Y, Krull UJ (2014) Lanthanide upconversion nanoparticles and applications in bioassays and bioimaging: a review. Anal Chim Acta 832:1–33
Baetke SC, Lammers T, Kiessling F (2015) Applications of nanoparticles for diagnosis and therapy of cancer. Br J Radiol 88:20150207
De Jong WH, Borm PJ (2008) Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine 3:133–149
Wang L, Zhao W, Tan W (2008) Bioconjugated silica nanoparticles: development and applications. Nano Res 1:99–115
Wolfbeis OS (2015) An overview of nanoparticles commonly used in fluorescent bioimaging. Chem Soc Rev 44:4743–4768
Nune SK, Gunda P, Thallapally PK, Lin Y-Y, Forrest ML, Berkland CJ (2009) Nanoparticles for biomedical imaging. Expert Opin Drug Deliv 6:1175–1194
Bannunah AM, Vllasaliu D, Lord J, Stolnik S (2014) Mechanisms of nanoparticle internalization and transport across an intestinal epithelial cell model: effect of size and surface charge. Mol Pharm 11:4363–4373
Lee S-C, Kim M-S, Yoo K-C, Ha N-R, Moon J-Y, Lee S-J, Yoon MY (2017) Sensitive fluorescent imaging of salmonella enteritidis and salmonella typhimurium using a polyvalent directed peptide polymer. Microchim Acta 184:2611–2620
El-Ansary A, Faddah LM (2010) Nanoparticles as biochemical sensors. Nanotechnol Sci Appl 3:65–76
Velusamy N, Binoy A, Bobba KN, Nedungadi D, Mishra N, Bhuniya S (2017) A bioorthogonal fluorescent probe for mitochondrial hydrogen sulfide: new strategy for cancer cell labeling. J Chem Soc Chem Commun 53:8802–8805
Zhang J, Yu S-H (2016) Carbon dots: large-scale synthesis, sensing and bioimaging. Mater Today 19:382–393
Li J, Jiao Y, Feng L, Zhong Y, Zuo G, Xie A, Dong W (2017) Highly n,p-doped carbon dots: rational design, photoluminescence and cellular imaging. Microchim Acta 184:2933–2940
Zheng M, Ruan S, Liu S, Sun T, Qu D, Zhao H, Xie Z, Gao H, Jing X, Sun Z (2015) Self-targeting fluorescent carbon dots for diagnosis of brain Cancer cells. ACS Nano 9:11455–11461
Wang D, Wang Z, Zhan Q, Pu Y, Wang J-X, Foster NR, Dai L (2017) Facile and scalable preparation of fluorescent carbon dots for multifunctional applications. Engineering 3:402–408
Qu K, Wang J, Ren J, Qu X (2013) Carbon dots prepared by hydrothermal treatment of dopamine as an effective fluorescent sensing platform for the label-free detection of iron (iii) ions and dopamine. Chem Eur J 19:7243–7249
Wang Q, Liu X, Zhang L, Lv Y (2012) Microwave-assisted synthesis of carbon nanodots through an eggshell membrane and their fluorescent application. Analyst 137:5392–5397
Zhou J, Sheng Z, Han H, Zou M, Li C (2012) Facile synthesis of fluorescent carbon dots using watermelon peel as a carbon source. Mater Lett 66:222–224
Sahu S, Behera B, Maiti TK, Mohapatra S (2012) Simple one-step synthesis of highly luminescent carbon dots from orange juice: application as excellent bio-imaging agents. J Chem Soc Chem Commun 48:8835–8837
Das P, Bose M, Ganguly S, Mondal S, Das AK, Banerjee S, Das NC (2017) Green approach to photoluminescent carbon dots for imaging of gram-negative bacteria Escherichia coli. Nanotechnology 28:195501
Xu H, Yang X, Li G, Zhao C, Liao X (2015) Green synthesis of fluorescent carbon dots for selective detection of Tartrazine in food samples. J Agric Food Chem 63:6707–6714
Kalytchuk S, Polkov K, Wang Y, Froning JP, Cepe K, Rogach AL, Zboil R (2017) Carbon dot Nanothermometry: intracellular photoluminescence lifetime thermal sensing. ACS Nano 11:1432–1442
Cui X, Wang Y, Liu J, Yang Q, Zhang B, Gao Y, Wang Y, Lu G (2017) Dual functional N- and S-co-doped carbon dots as the sensor for temperature and Fe3+ ions. Sens Actuator B-Chem 242:1272–1280
Shen C, Wang J, Cao Y, Lu Y (2015) Facile access to B-doped solid-state fluorescent carbon dots toward light emitting devices and cell imaging agents. J Mater Chem C 3:6668–6675
Bao R, Chen Z, Zhao Z, Sun X, Zhang J, Hou L, Yuan C (2018) Green and facile synthesis of nitrogen and phosphorus co-doped carbon quantum dots towards fluorescent ink and sensing applications. Nanomaterials 8:386
Xu Y, Li D, Liu M, Niu F, Liu J, Wang E (2017) Enhanced-quantum yield sulfur/nitrogen co-doped fluorescent carbon nanodots produced from biomass Enteromorpha prolifera: synthesis, posttreatment, applications and mechanism study. Sci Rep 7:4499
Sun Y, Shen C, Wang J, Lu Y (2015) Facile synthesis of biocompatible n, s-doped carbon dots for cell imaging and ion detecting. RSC Adv 5:16368–16375
Bhushan B, Kumar SU, Gopinath P (2016) Multifunctional carbon dots as effcient fluorescent nanotags for tracking cells through successive generations. J Mater Chem B 4:4862–4871
Zhai X, Zhang P, Liu C, Bai T, Li W, Dai L, Liu W (2012) Highly luminescent carbon nanodots by microwave-assisted pyrolysis. J Chem Soc Chem Commun 48:7955–7957
Meiling TT, Cywiski PJ, Bald I (2016) White carbon: fluorescent carbon nanoparticles with tunable quantum yield in a reproducible green synthesis. Sci Rep 6:28557
Li Z, Lu C, Xia Z, Zhou Y, Luo Z (2007) X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 45:1686–1695
Rajkumari J, Singha LP, Pandey P (2018) Genomic insights of aromatic hydrocarbon degrading klebsiella pneumoniae awd5 with plant growth promoting attributes: a paradigm of soil isolate with elements of biodegradation. 3 Biotech 8:118
Shen J, Zhang T, Cai Y, Chen X, Shang S, Li J (2017) Highly fluorescent N,S-co-doped carbon dots: synthesis and multiple applications. New J Chem 41:11125–11137
Chen J, Liu J, Li J, Xu L, Qiao Y (2017) One-pot synthesis of nitrogen and sulfur co-doped carbon dots and its application for sensor and multicolor cellular imaging. J Colloid Interface Sci 485:167–174
Chai L, Zhou J, Feng H, Tang C, Huang Y, Qian Z (2015) Functionalized carbon quantum dots with dopamine for Tyrosinase activity monitoring and inhibitor screening: in vitro and intracellular investigation. ACS Appl Mater Interfaces 7:23564–23574
Acknowledgements
The authors gratefully acknowledge the Department of Biotechnology (DBT), Govt. of India for the financial support through the project no. 102SAN/2237/2016-2017 and project no. 102/IFD/SAN/1409/2018-2019. The authors would like to thank Sophisticated Analytical Instruments Facility (SAIF), Cochin University of Science and Technology (CUSAT) for their unsparing support in HR-TEM image acquisition. The authors would also like to thank Mr. Jayesh Vasudeva Adhikari (University of Southern California), Mr. Prateek Katare (Indian Institute of Science, Bangalore) and Ms. Divya Nair (Amrita School of Biotechnology, Kollam) for their generous help in providing the presentable schematics, MATLAB codes for particle size estimation and several other characterization techniques.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 12935 kb)
Rights and permissions
About this article
Cite this article
Pathak, A., PV, S., Stanley, J. et al. Multicolor emitting N/S-doped carbon dots as a fluorescent probe for imaging pathogenic bacteria and human buccal epithelial cells. Microchim Acta 186, 157 (2019). https://doi.org/10.1007/s00604-019-3270-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3270-7