Abstract
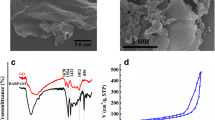
A nanocomposite (designated as PAG) possessing amphiphilic properties was prepared by a single-step method from graphene oxide and phytic acid, in which phytic acid acts as both an inducer and cross-linking agent. The morphology and microstructure of PAG were characterized by nitrogen adsorption, scanning electron microscopy and transmission electron microscopy. The PAG possess a 3 dimensional network structure with interpenetrated nano- and micropores and represent a viable adsorbent for solid-phase extraction of carbamate pesticides prior to their quantitation by high performance liquid chromatography. Under optimum conditions, the calibration plot is linear in the 0.5 to 80 ng g−1 concentration range in case of apple samples, and in the 1.0 to 80 ng mL−1 range for juice samples. The respective limits of detection (for S/N = 3) are between 0.05 and 0.1 ng g−1, and between 0.2 and 0.3 ng mL−1. The PAG has a high adsorption capability, and in our preception it may become a useful adsorbent for the preconcentration of other organic pollutants.

A amphiphilic nanocomposite (prepared from phytic acid and graphene oxide; denoted as PAG) was prepared by a single-step method. Phytic acid acts as both an inducer and cross-linking agent. The PAG was used as an adsorbent to extract carbamates from apple and juice samples.





Similar content being viewed by others
References
Li N, Chen J, Shi YP (2015) Magnetic graphene solid-phase extraction for the determination of carbamate pesticides in tomatoes coupled with high performance liquid chromatography. Talanta 141:212–219
Zhao G, Wang C, Wu Q, Wang Z (2011) Determination of carbamate pesticides in water and fruit samples using carbon nanotube reinforced hollow fiber liquid-phase microextraction followed by high performance liquid chromatography. Anal Chem 3:1410–1417
Ma X, Wang J, Wu Q, Wang C, Wang Z (2014) Extraction of carbamate pesticides in fruit samples by graphene reinforced hollow fibre liquid microextraction followed by high performance liquid chromatographic detection. Food Chem 157:119–124
He H, Xu X, Lu M, Mo W, Ren Y (2014) Determination of residues of carbamates and their metabolites in ginger by ultra-high performance liquid chromatography-tandem mass spectrometry. J Instrum Anal 33:197–202
Abad JM, Pariente F (1998) Determination of organophosphorus and carbamate pesticides using a piezoelectric biosensor. Anal Chem 70:2848–2855
Latrous El Atrache L, Ben Sghaier R, Bejaoui Kefi B, Haldys V, Dachraoui M, Tortajada J (2013) Factorial design optimization of experimental variables in preconcentration of carbamates pesticides in water samples using solid phase extraction and liquid chromatography-electrospray-mass spectrometry determination. Talanta 117:392–398
Gao L, Chen L, Li X (2014) Magnetic molecularly imprinted polymers based on carbon nanotubes for extraction of carbamates. Microchim Acta 182:781–787
Morais S, Dias E, Pereira ML (2012) "Carbamates: human exposure and health effects" in The Impact of Pesticides, Academy Publish, Cheyenne, Wyoming M. Jokanovic, Ed., pp. 21–38
Sagratini G, Manes J, Giardina D, Damiani P, Pico Y (2007) Analysis of carbamate and phenylurea pesticide residues in fruit juices by solid-phase microextraction and liquid chromatography-mass spectrometry. J Chromatogr A 1147:135–143
Lopez-Blanco MC, Cancho-Grande B, Simal-Gandara J (2002) Comparison of solid-phase extraction and solid-phase microextraction for carbofuran in water analyzed by high-performance liquid chromatography-photodiode-array detection. J Chromatogr A 963:117–123
Farajzadeh MA, Sorouraddin SM, Mogaddam MRA (2014) Liquid phase microextraction of pesticides: a review on current methods. Microchim Acta 181:829–851
Hao L, Liu X, Wang J, Wang C, Wu Q, Wang Z (2015) Use of ZIF-8-derived nanoporouscarbon as the adsorbent for the solid phase extraction of carbamate pesticides prior to high-performance liquid chromatographic analysis. Talanta 142:104–109
Liu X, Wang C, Wu Q, Wang Z (2015) Magnetic porous carbon-based solid-phase extraction of carbamates prior to HPLC analysis. Microchim Acta 183:415–421
Campanella B, Pulidori E, Onor M, Passaglia E, Tegli S, Izquierdo CG, Bramanti E (2016) New polymeric sorbent for the solid-phase extraction of indole-3-acetic acid from plants followed by liquid chromatography-fluorescence detector. Microchem J 128:68–74
Hennion M-C (1999) Solid-phase extraction: method development, sorbents, and coupling with liquid chromatography. J Chromatogr A 856:3–54
Zhao G, Wang C, Wu Q, Wang Z (2011) Determination of carbamate pesticides in water and fruit samples using carbon nanotube reinforced hollow fiber liquid-phase microextraction followed by high performance liquid chromatography. Anal Methods 3:1410–1417
Jenkins AL, Yin R, Jensen JL (2001) Molecularly imprinted polymer sensors for pesticide and insecticide detection in water. Analyst 126:798–802
Ahadi E, Hosseini-Monfared H, Mayer P (2015) Crystal structure of dichloridobis (methyl isonicotinate-κN) copper (II). Acta Cryst 71:m112–m113
De Smedt C, Biswas SP, Vandeput D, De Wilde T, Van Der Voort P, Spanoghe P (2012) Adsorption of pesticides on metal organic frameworks for water treatment. Commun Agric Appl Biol Sci 77:19–19
Liu J, Du H, Yuan S, He W, Liu Z (2015) Synthesis of thiol-functionalized magnetic graphene as adsorbent for Cd(II) removal from aqueous systems. J Environ Chem Eng 3:617–621
Wang F, Liu S, Yang H, Zheng J, Qiu J, Xu J, Tong Y, Zhu F, Ouyang G (2016) Hierarchical graphene coating for highly sensitive solid phase microextraction of organochlorine pesticides. Talanta 160:217–224
Allen MJ, Tung VC, Kaner RB (2010) Honeycomb carbon: A review of graphene. Chem Rev 110:132–145
Lv W, Zhang C, Li Z, Yang QH (2015) Self-assembled 3D graphene monolith from solution. J Phys Chem Lett 6:658–668
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Wu Y, Nguyen ST, Ruoff RS (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45:1558–1565
Cao X, Shi Y, Shi W, Lu G, Huang X, Yan Q, Zhang Q, Zhang H (2011) Preparation of novel 3D graphene networks for supercapacitor applications. Small 7:3163–3168
Shackery I, Patil U, Pezeshki A, Shinde NM, Im S, Jun SC (2016) Enhanced non-enzymatic amperometric sensing of glucose using Co(OH)2 nanorods deposited on a three dimensional graphene network as an electrode material. Microchim Acta 183:2473–2479
Sun L, Wang L, Tian C, Tan T, Xie Y, Shi K, Li M, Fu H (2012) Nitrogen-doped graphene with high nitrogen level via a one-step hydrothermal reaction of graphene oxide with urea for superior capacitive energy storage. RSC Adv 2:4498–4506
Chen W, Yan L (2011) In situ self-assembly of mild chemical reduction graphene for three-dimensional architectures. Nano 3:3132–3137
Song X, Chen Y, Rong M, Xie Z, Zhao T, Wang Y, Chen X, Wolfbeis OS (2016) A phytic acid induced super-amphiphilic multifunctional 3D graphene-based foam. Angew Chem Int Ed Engl 55:3936–3941
Wang C, Feng C, Gao Y, Ma X, Wu Q, Wang Z (2011) Preparation of a graphene-based magnetic nanocomposite for the removal of an organic dye from aqueous solution. Chem Eng J 173:92–97
Gao L, Zhang C, Zhang M, Huang X, Jiang X (2009) Phytic acid conversion coating on Mg-Li alloy. J Alloys Compd 485:789–793
Jiang G, Qiao J, Hong F (2012) Application of phosphoric acid and phytic acid-doped bacterial cellulose as novel proton-conducting membranes to PEMFC. Int J Hydrog Energy 37:9182–9192
Acknowledgments
Financial supports from the National Natural Science Foundation of China (31471643, 31571925, 31671930), the Natural Science Foundation of Hebei Province (B2016204136, B2016204146, B2017204025), the Scientific and Technological Research Foundation of the Department of Education of Hebei Province (ZD2016085), the Natural Science Foundation of Agricultural University of Hebei (LG201607) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human or animal subjects.
Electronic supplementary material
ESM 1
(DOC 1190 kb)
Rights and permissions
About this article
Cite this article
Wu, J., Liang, X., Hao, L. et al. Graphene oxide cross-linked with phytic acid: an efficient adsorbent for the extraction of carbamates. Microchim Acta 184, 3773–3779 (2017). https://doi.org/10.1007/s00604-017-2413-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2413-y