Abstract
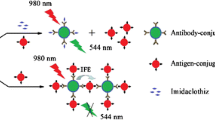
Cadmium-telluride quantum dots (QDs) were conjugated to an antibody (Ab) against Citrus tristeza virus (CTV), while the coat protein (CP) of the CTV was immobilized on the surface of carbon nanoparticles (CNPs). Following immunobinding of the QD-Ab and the CP-loaded CNPs, the fluorescence of the CdTe QDs was quenched by the CNPs. This effect was exploited to design a detection assay for the CTV which was found more sensitive and specific than the existing enzyme linked immunosorbent assay (ELISA). The limit of detection was measured at about 220 ng⋅ mL‾1 of CTV. The Stern-Volmer plot of the CNPs-QD quencher pair showed a positive deviation from linearity which was ascribed to the presence of both static and dynamic quenching.

Cadmium-telluride quantum dots (QDs) were conjugated to an antibody (Ab) against Citrus tristeza virus (CTV), while the coat protein (CP) of the CTV was immobilized on the surface of carbon nanoparticles (CNPs). Following immunobinding of the QD-Ab and the CP-loaded CNPs, the fluorescence of the CdTe QDs was quenched by the CNPs. This effect was exploited to design a detection assay for the CTV which was found more sensitive and specific than the existing enzyme linked immunosorbent assay (ELISA).






Similar content being viewed by others
References
Moreno P, Ambrós S, Albiach-Martí MR, et al. (2008) Citrus tristeza virus: a pathogen that changed the course of the citrus industry. Mol Plant Pathol 9:251–268. doi:10.1111/J.1364-3703.2007.00455.X
Bertolini E, Moreno A, Capote N, et al. (2008) Quantitative detection of citrus tristeza virus in plant tissues and single aphids by real-time RT-PCR. Eur J Plant Pathol 120:177–188. doi:10.1007/s10658-007-9206-9
Sankaran S, Mishra A, Ehsani R, Davis C (2010) A review of advanced techniques for detecting plant diseases. Comput Electron Agric 72:1–13. doi:10.1016/j.compag.2010.02.007
Loconsole G, Saponari M, Savino V (2010) Development of real-time PCR based assays for simultaneous and improved detection of citrus viruses. Eur J Plant Pathol 128:251–259. doi:10.1007/s10658-010-9653-6
Barbarossa L, Savino V (2006) Sensitive and specific digoxigenin-labelled RNA probes for routine detection of citrus tristeza virus by dot-blot hybridization. J Phytopathol 154:329–335. doi:10.1111/j.1439-0434.2006.01102.x
Garibotti AV, Pérez-Rentero S, Eritja R (2011) Functionalization and self-assembly of DNA bidimensional arrays. Int J Mol Sci 12:5641–5651. doi:10.3390/ijms12095641
Cerf A, Alava T, Barton RA, Craighead HG (2011) Transfer-printing of single DNA molecule arrays on graphene for high-resolution electron imaging and analysis. Nano Lett 11:4232–4238. doi:10.1021/nl202219w
Liu W, Zhong H, Wang R, Seeman NC (2011) Crystalline two-dimensional DNA-origami arrays. Angew Chem Int Ed Eng 50:264–267. doi:10.1002/anie.201005911
Hongyang S, Yalei Z, Chunmin Z, et al. (2011) Cultivation of Chlorella pyrenoidosa in soybean processing wastewater. Bioresour Technol 102:9884–9890. doi:10.1016/j.biortech.2011.08.016
Wu P, Li Y, Yan X-P (2009) CdTe quantum dots (QDs) based kinetic discrimination of Fe 2+ and Fe 3+, and CdTe QDs-Fenton hybrid system for sensitive photoluminescent detection of Fe 2 +. Anal Chem 81:6252–6257. doi:10.1021/ac900788w
Pan J, Feng S-S (2009) Targeting and imaging cancer cells by folate-decorated, quantum dots (QDs)- loaded nanoparticles of biodegradable polymers. Biomaterials 30:1176–1183. doi:10.1016/j.biomaterials.2008.10.039
Fei X, Gu Y, Wang J, et al. (2011) Preparation and fluorescent properties of a complex probe based on inorganic QDs and organic dye. J Lumin 131:291–296. doi:10.1016/j.jlumin.2010.10.015
Zuo P, Lu X, Sun Z, et al. (2015) A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim Acta 183:519–542. doi:10.1007/s00604-015-1705-3
Safarpour H, Safarnejad MR, Tabatabaei M, et al. (2012) Development of a quantum dots FRET-based biosensor for efficient detection of Polymyxa betae. Can J Plant Pathol 34:507–515. doi:10.1080/07060661.2012.709885
Zekavati R, Safi S, Hashemi SJ, et al. (2013) Highly sensitive FRET-based fluorescence immunoassay for aflatoxin B1 using cadmium telluride quantum dots. Microchim Acta 180:1217–1223. doi:10.1007/s00604-013-1047-y
Roodbar Shojaei T, Mohd Salleh MA, Tabatabaei M, et al. (2014) Development of sandwich-form biosensor to detect mycobacterium tuberculosis complex in clinical sputum specimens. Braz J Infect Dis 18:600–608. doi:10.1016/j.bjid.2014.05.015
Liu M, Zhao H, Quan X, et al. (2010) Distance-independent quenching of quantum dots by nanoscale-graphene in self-assembled sandwich immunoassay. Chem Commun 46:7909–7911. doi:10.1039/c0cc02085k
Cui D, Pan B, Zhang H, et al. (2008) Self-assembly of quantum dots and carbon nanotubes for ultrasensitive DNA and antigen detection. Anal Chem 80:7996–8001. doi:10.1021/ac800992m
Lakowicz JR (2006) Principles of fluorescence spectroscopy, 3rd ed. doi:10.1007/978–0–387-46312-4
Chao M-R, Hu C-W, Chen J-L (2014) Comparative syntheses of tetracycline-imprinted polymeric silicate and acrylate on CdTe quantum dots as fluorescent sensors. Biosens Bioelectron 61:471–477. doi:10.1016/j.bios.2014.05.058
Mohd Yazid SNA, Chin SF, Pang SC, Ng SM (2013) Detection of Sn(II) ions via quenching of the fluorescence of carbon nanodots. Microchim Acta 180:137–143. doi:10.1007/s00604-012-0908-0
Al-Kady AS, Gaber M, Hussein MM, Ebeid E-ZM (2011) Structural and fluorescence quenching characterization of hematite nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc 83:398–405. doi:10.1016/j.saa.2011.08.052
Nemzek TL (1975) Kinetics of diffusion-controlled reactions: transient effects in fluorescence quenching. J Chem Phys 62:477–489. doi:10.1063/1.430501
Bhunia SK, Saha A, Maity AR, et al. (2013) Carbon nanoparticle-based fluorescent bioimaging probes. Sci Rep 3:1473–1479. doi:10.1038/srep01473
Zhu A, Qu Q, Shao X, et al. (2012) Carbon-dot-based dual-emission nanohybrid produces a ratiometric fluorescent sensor for in vivo imaging of cellular copper ions. Angew Chem Int Ed Eng 51:7185–7189. doi:10.1002/anie.201109089
Adkar-Purushothama CR, Maheshwar PK, Sano T, Janardhana GR (2011) A sensitive and reliable RT-nested PCR assay for detection of citrus tristeza virus from naturally infected citrus plants. Curr Microbiol 62:1455–1459. doi:10.1007/s00284-011-9883-7
Mathews DM, Riley K, Dodds JA (1997) Comparison of detection methods for citrus tristeza virus in field trees during months of nonoptimal titer. Plant Dis 81:525–529. doi:10.1094/PDIS.1997.81.5.525
Zhang S, He Q, Li R, et al. (2011) Study on the fluorescence carbon nanoparticles. Mater Lett 65:2371–2373. doi:10.1016/j.matlet.2011.05.025
Yang R, Tang Z, Yan J, et al. (2008) Noncovalent assembly of carbon nanotubes and single-stranded DNA: an effective sensing platform for probing biomolecular interactions. Anal Chem 80:7408–7413. doi:10.1021/ac801118p
Morales-Narváez E, Pérez-López B, Pires LB, Merkoçi A (2012) Simple förster resonance energy transfer evidence for the ultrahigh quantum dot quenching efficiency by graphene oxide compared to other carbon structures. Carbon N Y 50:2987–2993
Ma N, Jiang W, Li T, et al. (2015) Fluorescence aggregation assay for the protein biomarker mucin 1 using carbon dot-labeled antibodies and aptamers. Microchim Acta 182:443–447. doi:10.1007/s00604-014-1386-3
Du F, Zeng F, Ming Y, Wu S (2013) Carbon dots-based fluorescent probes for sensitive and selective detection of iodide. Microchim Acta 180:453–460. doi:10.1007/s00604-013-0954-2
Wang Y, Bao L, Liu Z, Pang D-W (2011) Aptamer biosensor based on fluorescence resonance energy transfer from upconverting phosphors to carbon nanoparticles for thrombin detection in human plasma. Anal Chem 83:8130–8137. doi:10.1021/ac201631b
Evale BG, Hanagodimath SM (2009) Fluorescence quenching of newly synthesized biologically active coumarin derivative by aniline in binary solvent mixtures. J Lumin 129:1174–1180. doi:10.1016/j.jlumin.2009.05.017
Melavanki RM, Kusanur RA, Kadadevaramath JS, Kulakarni MV (2009) Quenching mechanisms of 5BAMC by aniline in different solvents using stern–Volmer plots. J Lumin 129:1298–1303. doi:10.1016/j.jlumin.2009.06.011
Biradar DS, Thipperudrappa J, Hanagodimath SM (2007) Fluorescence quenching of 2,2″ dimethyl-p-terphenyl by carbon tetrachloride in different solvents and temperatures. J Lumin 126:339–346. doi:10.1016/j.jlumin.2006.08.066
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
ESM 1
(DOC 95 kb)
Rights and permissions
About this article
Cite this article
Shojaei, T.R., Salleh, M.A.M., Sijam, K. et al. Fluorometric immunoassay for detecting the plant virus Citrus tristeza using carbon nanoparticles acting as quenchers and antibodies labeled with CdTe quantum dots. Microchim Acta 183, 2277–2287 (2016). https://doi.org/10.1007/s00604-016-1867-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1867-7