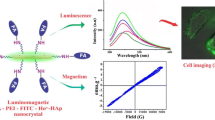
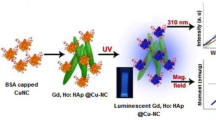
Abstract
Hydroxyapatite (HAp) is the most important constituent of biological tissues such as bone and teeth and exhibits several characteristic features. HAp nanoparticles (NPs) are good host materials and can be functionalized with various kinds of dopants and substrates. By endowing HAp NPs with desired properties in order to render them suitable for biomedical applications including cellular imaging, non-invasive and quantitative visualisation of molecular process occurring at cellular and subcellular levels becomes possible. Depending on their functional properties, HAp based nanoprobes can be divided into three classes, i.e., luminescent HAp NPs (for both downconversion and upconversion luminescence), magnetic HAp NPs, and luminomagnetic HAp NPs. Luminomagnetic HAp NPs are particularly attractive in terms of bimodal imaging and even multimodal imaging by virtue of their luminescence and magnetism. Functionalised HAp NPs are potential candidates for targeted drug delivery applications. This review (with 166 references) spotlights the cellular imaging applications of three types of HAp NPs. Specific sections cover aspects of molecular imaging and the various imaging modes, a comparison of the common types of nanoprobes for bioimaging, synthetic methods for making the various kinds of HAp NPs, followed by overviews on fluorescent NPs for bioimaging (such as quantum dots, gold nanoclusters, lanthanide-doped or fluorophore-doped NPs), magnetic HAp NPs for use in magnetic resonance imaging (MRI), luminomagnetic HAp NPs for bimodal imaging, and sections on drug delivery as well as cellular imaging applications of HAp based nanoprobes (including targeted imaging).

Hydroxyapatite nanoparticles (HAp NPs) with different functional properties such as luminescence and magnetism are potential candidates for drug delivery as well as multimodal imaging. The review spotlights such applications of luminescent, magnetic and luminomagnetic HAp NPs and discussed their synthesis and characterization.









Similar content being viewed by others
References
Hui J, Wang X (2014) Hydroxyapatite nanocrystals: colloidal chemistry, assembly and their biological applications. Inorg Chem Front 1:215–225
Meyers MA, Chen PY, Lin AYM, Seki Y (2008) Biological materials: structure and mechanical properties. Prog Mater Sci 53:1–206
Palmer LC, Newcomb CJ, Kaltz SR, Spoerke ED, Stupp SI (2008) Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem Rev 108:4754–4783
Stigter M, Groot K, Layrolle P (2002) Incorporation of tobramycin into biomimetic hydroxyapatite coating on titanium. Biomaterials 23:4143–4153
Fox K, Tran PA, Tran N (2012) Recent advances in research applications of nanophase hydroxyapatite. Chem Phys Chem 13:2495–2506
Lafisco M, Delgado-Lopez JM, Varoni EM, Tampieri A, Rimondini L, Gomez-Morales J, Prat M (2013) Cell surface receptor targeted biomimetic apatite nanocrystals for cancer therapy. Small 25:3834–3844
Xu J, White T, Li P, He C, Han Y-F (2010) Hydroxyapatite foam as a catalyst for formaldehyde combustion at room temperature. J Am Chem Soc 132:13172–13173
Liu TY, Chen SY, Liu DM, Liou SC (2005) On the study of BSA-loaded calcium-deficient hydroxyapatite nano-carriers for controlled drug delivery. J Control Release 107:112–121
Uskovic V, Uskovic DP (2011) Nanosized hydroxyapatite and other calcium phosphates: chemistry of formation and application as drug and gene delivery agents. J Biomed Mater Res B 96B:152–191
Luo Y, Ling Y, Guo W, Pang J, Liu W, Fang Y, Wen X, Wei K, Gao X (2010) Docetaxel loaded oleic acid-coated hydroxyapatite nanoparticles enhance the docetaxel-induced apoptosis through activation of caspase-2 in androgen independent prostate cancer cells. J Control Release 147:278–288
Hou C H, Hou S M, Hsueh Y S, Lin J, Wu H C, Lin F H The in vivo performance of biomagnetic hydroxyapatite nanoparticles in cancer hyperthermia therapy. Biomaterials 30:3956–3960
Li J, Yin Y, Yao F, Zhang L, Yao K (2008) Effect of nano-and micro-hydroxyapatite/chitosan-gelatin network film on human gastric cancer cells. Mater Lett 62:3220–3223
Nazari AG, Tahari A, Moztarzadeh M, Mozafari M, Bahroloom ME (2011) Ion exchange behaviour of silver-doped apatite micro and nanoparticles as antibacterial biomaterial. Micro Nano Lett 6:713–717
Radovanovic Z, Jokic B, Veljovic D, Dimitrijevic S, Kojic V, Petrovic R, Janackovic D (2014) Antimicrobial activity and biocompatibility of Ag+ - and Cu2+ - doped biphasic hydroxyapatite/ α-tricalcium phosphate obtained from hydrothermally synthesized Ag+ - and Cu2+ - doped hydroxyapatite. Appl Surf Sci 307:513–519
Remya NS, Syama S, Gayathri V, Varma HK, Mohanan PV (2014) An in vitro study on the interaction of hydroxyapatite nanoparticles and bone marrow mesenchymal stem cells for assessing the toxicological behaviour. Colloids Surf B 117:389–397
Byrne JD, Betancourt T, Brannon-Peppas L (2008) Active targeting schemes for nanoparticle system in cancer therapeutics. Adv Drug Deliv Rev 60:1615–1626
Wang SG, Li N, Pan W, Tang B (2012) Advances in functional fluorescent and luminescent probes for imaging intracellular small-molecular reactive species. Trends Anal Chem 39:3–37
Niu J, Wang X, Lv J, Li Y, Tang B (2014) Luminescent nanoprobes for in-vivo bioimaging. Trend Anal Chem 58:112–119
Kim J, Piao Y, Hyeon Y (2008) Multifunctional nanostructured materials for multimodel imaging, and simultaneous imaging and therapy. Chem Soc Rev 38:372–390
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R (2007) Nanocarrers as an emerging platform for cancer therapy. Nat Nanotechnol 12:751–760
Lee SY, Jeon SI, Jung SH, Chung IJ, Ahn C-H (2014) Targeted multimodal imaging modalities. Adv Drug Deliv Rev 76:60–78
Norek M, Peters AJ (2011) MRI contrast agents on dysprosium or holmium. Prog Nucl Magn Reson Spectrosc 59:64–82
Xi D, Dong S, Meng X, Lu Q, Meng L, Ye J (2012) Gold nanoparticles as computerized tomography (CT) contrast agents. RSC Adv 2:12515–12524
Lusic H, Grinstaff MW (2013) X-ray-computed tomography contrast agents. Chem Rev 113:1641–1666
Ametamy SM, Honer M, Schubiger PA (2008) Molecular imaging with PET. Chem Rev 108:1501–1516
Li Z, Conti PS (2010) Radiopharmaceutical chemistry for positron emission tomography. Adv Drug Deliv Rev 62:1031–1051
Wades TJ, Wong EH, Weisman GR, Anderson CJ (2010) Coordinating radiometals of copper, gallium, indium, yttrium and zirconium for PET and SPECT imaging of disease. Chem Rev 110:2858–2902
Gobin AM, Lee MH, Halas NJ, James WD, Drezek RA, Weast JL (2007) Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett 7:1929–1934
Fercher AF (2009) Optical coherence tomography-development, principles, applications. Z Med Phys 20:251–276
Pansare VJ, Hejazi S, Faenza W, Prudehomme RK (2012) Review of long-wavelength optical and NIR imaging materials: ontrast agents, fluorophores, and multifunctional nano carriers. Chem Mater 24:812–827
Harvey CJ, Blomley MJK, Eckersley RJE, Cosgrove DO (2001) Develpoements in ultrasound contrast media. Eur Radiol 11:675–689
Guo C, Jin Y, Dai Z (2014) Multifunctional ultrasound contrast agents for imaging guided photothermal therapy. Bioconjug Chem 25:840–854
Lu W, Huang Q, Ku G, Wen X, Zhou M, Guzatov D, Brecht P, Su R, Oraevsky A, Wang LV, Li C (2010) Photoacoustic imaging of living mouse brain vasculature using hollow gold nanospheres. Biomaterials 31:2617–2626
Savita N, Maitra S, Ravishankar U (2010) Multimodality molecular imaging – an overview with special focus on PET/CT. Apollo Med 7:190–199
Jennings L E, Long N J (2009) Two is better than one-probes for dual-modality molecular imaging. Chem Commun 3511–3524
Frullano L, Meade TJ (2007) Multimodal MRI contrast agents. J Biol Inorg Chem 12:939–949
Jadvar H, Colletti PM (2014) Competitive advantage of PET/MR. Eur J Radiol 83:84–94
Pichler B, Wehrl HF, Kolb A, Judenhofer MS (2008) Positron emission tomography/ magnetic resonance imaging: the next generation of multimodality imaging? Semin Nucl Med 38:199–208
Debasu ML, Ananias D, Pinho SLC, Geraldes CFGC, Carlos LD, Rocha J (2012) (Gd, Yb, Tb)PO4 up -conversion nanocrystals for bimodal luminescence- MR imaging. Nanoscale 4:5154–5162
Qiao Z, Shi X (2014) Dendrimer-based molecular imaging contrast agents. Prog Polym Sci. doi:10.1016/j.progpolymsci.2014.08.002
Wolbeis O S (2015) An overview of nanoparticles commonly used in fluorescent bioimaging. doi:10.1039/c4cs00392f
Bae SW, Tan W, Hong J (2012) Fluorescent dye-doped silica nanoparticles: new tools for bioapplications. Chem Commun 48:2270–2282
Wang X, Meier RJ, Wolfbis OS (2013) Fluorescent pH-sensitive nanoparticles in an agarose matrix for imaging of bacterial growth and metabolism. Angew Chem Int Ed 52:406–409
Tang R, Feng X (2014) Highly luminescent conjugated polymer nanoparticles for imaging and therapy. Can Chem Trans 1:78–84
Song Y, Zhu S, Yang B (2014) Bioimaging based on fluorescent carbon dots. RSC Adv 4:27184–27200
Yang Y (2014) Upconversion nanophosphores for use in bioimaging, therapy, drug delivery and bioassays. Microchim Acta 181:263–294
Cui M, Zhao Y, Song Q (2014) Synthesis, optical properties and applications of ultra-small luminescent gold nanoclusters. Trends Anal Chem 57:73–82
Caravan P, Ellison JJ, McMurry TJ, Lauffer RB (1999) Gadollinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem Rev 99:2293–2352
Nicolay K, Strijkers G, Grull H (2013) Gd- Containing nanoparticles as MRI contrast agents. In: Merbach A (ed) The chemistry of contrast agents in medical magnetic resonance imaging, 2nd edn. Wiley, UK, pp 449–483
Sharma P, Brown S, Walter G, Santra S, Moudgil B (2006) Nanoparticles for bioimaging. Adv Colloid Interf Sci 123:471–485
Lu X, Leng Y (2005) Theoretical analysis of calcium phosphate precipitation in simulated body fluid. Biomaterials 26:1097–1108
Sadat-Shojai M, Khorasani M, Dinpanah-Khoshdargi E, Jamshidi A (2013) Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater 9:7591–7621
Liu D-M, Troczynski T, Tseng WJ (2001) Water-based sol–gel synthesis of hydroxyapatite:process development. Biomaterials 22:1721–1730
Salimi MN, Anuar A (2013) Charecterizations of biocompatible and bioactive hydroxyapatite particles. Procedia Eng 53:192–196
Wang J, Shaw L L (2009) Synthesis of high purity hydroxyapatite nanopowder via sol–gel combustion process. 20: 1223–1227
Han Y, Li S, Wang X, Chen X (2004) Synthesis and sintering of nanocrystalline hydroxyapatite powders by citric acid sol–gel combustion method. Mater Res Bull 39:25–32
Ruksudjarit A, Pengpat K, Rujijanagul G, Tunkasiri T (2008) Synthesis and characterization of nanocrystalline hydroxyapatite from natural bovine bone. Curr Appl Phys 8:270–272
Tabakovic A, Kester M, Adair JH (2012) Calcium phosphate- based composite nanoparticles in bioimaging and therapeutic delivery applications. WIREs Nanomed Nanobiotechnol 4:96–112
Talapin DV, Gaponik N, Borchert H, Rogach AL, Hasse M, Weller H (2002) Etching of colloidal InP nanocrystal with fluorides: photochemical nature of the process resulting in high photoluminescence efficiency. J Phys Chem B 106:12659–12663
Derfus AM, Chan WCW, Bhatia SN (2003) Probing the cytotoxicity of semiconductor quantum dots. Nano Lett 4:11–18
Zhang J, Fatouros PP, Shu C, Reid J, Qwens LS, Cai T, Gibson HW, Long GL, Corwin FD, Chen ZJ, Dorn HC (2010) High relaxivity trimetallic nitride (Gd3N) metallofullerene MRI contrast agents with optimized functionality. Bioconjug Chem 21:610–615
Bunzil JG (2010) Lanthanide luminescence for biomedical analyse and imaging. Chem Rev 110:2729–2755
Bunzli J-CG, Eliseeva SV (2011) Basics of lanthanide photophysics. In: Haenninen P, Haerma H (eds) Lanthanide Luminescence: Photophysical, Analytical and Biological Aspects, Springer series on Fluorescence, vol 7. Springer, Heidelberg, pp 1–46
Eliseeva SV, Bunzil JG (2009) Lanthanide luminescence for functional materials and bio-sciences. Chem Soc Rev 39:189–227
Zhang Y, Wei W, Das GK, Tan TTY (2014) Engineering lanthanide-based materials for nanomedicine. J Photochem Photobiol C 20:71–96
Werts MHV (2005) Making sense of lanthanide luminescence. Sci Prog 88:101–131
Bunzil JC (2006) Benefiting from the unique properties of lanthanide ions. Acc Chem Res 39:53–61
Montogomery C P, Murray B S, New E J, Pal R, Parker D (2009) Cell-penetrating metal complex optical probes: targeted and responsive systems based on lanthanide luminescence. 42: 925–937
Wang S, Wang L Lanthanide-doped nanomaterials for luminescence detection and imaging. doi:10.1016/j.trac.2014.07.011
Fang Y, Xu A, Song R, Zhang H, You L, Yu J, Liu H (2003) Systematic synthesis and characterization of single-crystal lanthanide orthophosphate nanowires. J Am Chem Soc 125:16025–16034
Li C, Quan Z, Yang J, Lin J (2007) Highly uniform and monodisperse β-NaYF4:Ln3 + (Ln = Eu, Tb, Yb/Er, and Yb/Tm) hexagonal microprism crystals: hydrothermal synthesis and luminescent properties. Inorg Chem 46:6329–6337
Wegh RT, Donker H, Oskam KD, Meijerink (1999) Visible quantum cutting in LiGdF4: Eu3+ through downconversion. Science 283:663–666
Mader HS, Kele P, Saleh SM, Wolfbeis OS (2010) Upconverting luminescent nanoparticles for use in biocojugation and bioimaging. Curr Opin Chem Biol 14:582–596
Chen G, Qiu H, Prasad PN, Chen X (2014) Upconversion nanoparticles: design, nanochemistry and applications in theranostics. Chem Rev 114:5161–5214
Weng M, Abbinei G, Clevenger A, Mao C, Xu S (2011) Upconversion nanoparticles: synthesis, surface modification and biological applications. Nanomed: Nanotechnol Biol Med 7:710–729
Auzel F (2004) Upconversion and anti-stokes processes with f and d ions in solids. Chem Rev 104:139–173
Heffern MC, Matosziuk LM, Meade TJ (2014) Lanthanide probes for bioresponsive imaging. Chem Rev 114:4496–4539
Doat A, Fanjul M, Pelle F, Hollande E, Lebugle A (2003) Europium-doped bioapatite: a new photostable biological probe, internalizable by human cells. Biomaterials 24:3365–3371
Ternane R, Ayedi MT, Ariguib NK, Piriou B (1999) Luminescent properties of Eu3+ in calcium hydroxyapatite. J Lumin 81:165–170
Cao XY, Wen F, Bian W, Cao Y, Pang C, Zhang W (2009) Preparation and comparison study of hydroxyl apatite and Eu-hydroxyapatite. Front Mater Sci 3:255–258
Kim EJ, Choi S, Hong S (2007) Synthesis and photoluminescence properties of Eu3+ -doped calcium phosphates. J Am Ceram Soc 9:2795–2798
Huang S, Zhu J, Zhou K (2012) Effects of Eu3+ ions on the morphology and luminescence properties of hydroxyapatite nanoparticles synthesized by one-step hydrothermal method. Mater Res Bull 47:24–28. doi:10.1016/j.materresbull.2011.10.013
Long M, Hong F, Li W, Zhao H, Lv Y, Li H, Hu F, Sun L, Yan C, Wei Z (2008) Size-dependent microstructure and europium site preference influence fluorescent properties of Eu3+-doped Ca10(PO4)6(OH)2 nanocrystal. J Lumin 128:428–436
Andre RS, Paris EC, Gurgel MFC, Rosa ILV, Paiva-Santos CO, Li MS, Varela JA, Longo E (2012) Structural evolution of Eu-doped hydroxyapatite nanorods monitored photoluminescence emission. J Alloys Comp 531:50–54
Liu Y, Tu D, Zhu H, Chen X (2013) Lanthanide-doped luminescent nanoprobes: controlled synthesis, optical spectroscopy, and bioapplications. Chem Soc Rev 42:6924–6959
Feng Z, Li Y, Huang Y, Seo HJ (2011) Luminescence properties of Eu2+ and Eu3+ doped calcium- deficient hydroxyapatite prepared in air. J Alloys Comp 509:7087–7092
Graeve OA, Kanakala R, Madadi A, Williams BC, Glass KC (2010) Luminescence variations in hydroxyapatites doped with Eu2+ and Eu3+ ions. Biomaterials 31:4259–4267
Sun Y, Yang H, Tao D (2012) Preparation and characterization of Eu3+ doped fluorapatite nanoparticles by a hydrothermal method. Ceram Int 38:6937–6941
Zhao Y, Zhu J, Zhu S, Huang Y, Li Z, Zhou K (2011) Synthesis and characterization of arginine modified europium-doped hydroxyapatite nanoparticle and its cell viability. Trans Nonferrous Metals Soc China 21:1773–1778
Wilusz RJ, Bednarkiewicz A, Strek W (2011) Synthesis and optical properties of Eu3+ ion doped nanocrystalline hydroxyapatite embedded in PMMA matrix. J Rare Earths 29:1111–1116
Popa CL, Ciobanu CS, Iconaru SL, Stan M, Dinischiotu A, Negrila CC, Heino MM, Guegan R, Predoi D (2014) Systematic investigation and in vitro biocompatibility studies on mesoporous europium doped hydroxyapatite. Cent Eur J Chem 12:1032–1046
Kattan AA, Dufour P, Ghys JD, Drouet C (2010) Preparation and physicochemical characteristics of luminescent apatite-based colloids. J Phys Chem C 114:2918–2924
Han Y, Wang X, Dai H, Li S (2013) Synthesis and luminescence of Eu3+ doped hydroxyapatite nanocrystallines: effects of calcinations and Eu3+ content. J Lumin 135:281–287
Hasna K, Kumar SS, Komath M, Varma MR, Jayaraj MK, Kumar KR (2013) Synthesis of chemically pure, luminescent Eu3+ doped Hap nanoparticles: a promising fluorescent probe for in vivo imaging applications. Phys Chem Chem Phys 15:8106–8111
Wagner DE, Eisenmann KM, Nester-Kalinoski AL, Bhaduri SB (2013) A microwave-assisted solution combustion synthesis to produce europium-doped calcium phosphate nanowhiskers for bioimaging applications. Acta Biomater 9:8422–8432
Yang P, Quan Z, Li C, Kang X, Lian H, Lin J (2008) Bioactive, luminescent and mesoporous europium-doped hydroxyapatite as drug carrier. Biomaterials 29:4341–4347
Yan-zhong Z, Yan-yan H, Jun Z, Shai-hong Z, Zhi-you L, Ke-chao Z (2011) Characteristics of functionalized nano-hydroxyapatite and internalization by human epithelial cell. Nanoscale Res Lett 6:600–607
Sun R, Chen K, Wu X, Zhao D, Sun Z (2013) Controlled synthesis and enhanced luminescence of europium -doped fluorine-substituted hydroxyapatite nanoparticles. Cryst Eng Comm 15:3442–3447
Escudero A, Calvo ME, Fernandez SR, de la Fuente JM, Ocana M (2013) Microwave-assisted synthesis of biocompatible europium-doped calcium hydroxyapatite and fluoroapatite luminescent nanospindles functionalized with poly(acrylic acid). Langmuir 29:1985–1994
Doat A, Pelle F, Gardant N, Lebugle A (2004) Synthesis of luminescent bioapatite nanoparticles for utilization as a biological probe. J Solid State Chem 177:1179–1187
Chen F, Zhu Y-J, Zhang K-H, Wu J, Wang K-W, Tang Q-L, Mo X-M (2011) Europium-doped amorphous calcium phosphate porous nanospheres: preparation and application as luminescent drug carriers. Nanoscale Res Lett 6:67–75
Dembski S, Milde M, Dyrba M, Schweizer S, Gellermann C, Klockenbring T (2011) Effect of pH on the synthesis and properties of luminescent SiO2/calcium phosphate: Eu3+ core-shell nanoparticles. Langmuir 27:14025–14032
Li X, Zeng H, Teng L, Chen H (2014) Comparative investigation on the crystal structure and cell behavior of rare-earth doped fluorescent apatite nanocrystals. Mater Lett 125:78–81
Li L, Liu Y, Tao J, Zhang M, Pan H, Xu X, Tang R (2008) Surface modification of hydroxyapatite nanocrystallite by a small amount of terbium provides a biocompatible fluorescent probe. J Phys Chem C 112:12219–12224
Yang C, Yang P, Wang W, Wang J, Zhang M, Lin J (2008) Solvothermal synthesis and charecterization of Ln (Eu3+, Tb3+) doped hydroxyapatite. J Colloid Interface Sci 328:203–210
Hui J, Zhang X, Zhang Z, Wang S, Tao L, Wei Y, Wang X (2012) Fluoridated HAp : Ln3+ (Ln = Eu or Tb) nanoparticles for cell imaging. Nanoscale 4:6967–6970
Lebugle A, Pelle F, Charvillat C, Rousselot I, Chane-Ching J Y (2006) Colloidal and monocrystalline Ln3+ doped apatite calcium phosphate as biocompatible fluorescent probes. Chem Commun 606–608
Zhang X, Hui J, Yang B, Yang Y, Fan D, Liu M, Tao L, Wei Y (2013) PEGylation of fluorinated hydroxyapatite (FAp):Ln3+ nanorods for cell imaging. Polym Chem 4:4120–4125
Neumeier M, Halis LA, Davis SA, Mann S, Epple M (2011) Synthesis of fluorescent core-shell hydroxyapatite nanoparticle. J Mater Chem 21:1250–1254
Sun Y, Yang H, Tao D (2011) Microemulsion process synthesis of lanthanide-doped hydroxyapatite nanoparticles under hydrothermal treatment. Ceram Int 37:2917–2920
Liu H, Xi P, Xie G, Chen F, Li Z, Bai D, Zeng Z (2011) Biocompatible hydroxyapatite nanoparticles as a redox luminescence switch. J Biol Inorg Chem 16:1135–1140
de Araujo T S, Macedo Z S, de Oliveira P A S C, Valerio M E G (2007) Production and Characterization of pure and Cr3+- doped hydroxyapatite for biomedical applications as fluorescent probes. 42: 2236–2243
Victor SP, Paul W, Jayabalan M, Sharma CP (2014) Supramolecular hydroxyapatite complexes as theranostic near-infrared luminescent drug carriers. Cryst Eng Comm 16:9033–9042
Liu H, Chen F, Xi P, Chen B, Huang L, Cheng J, Shao C, Wang J, Bai D, Zeng Z (2011) Biocompatible fluorescent hydroxyapatite: synthesis and live cell imaging applications. J Phys Chem C 115:18538–18544
Ge X, Li C, Fan C, Feng X, Cao B (2013) Enhanced photoluminescence properties of methylene blue dye encapsulated in nanosized hyroyapatite/silica particles with core-shell structure. Appl Phys A 113:583–589
Morgan TT, Mudddana HS, Altinoglu EI, Rouse SM, Tabakovic A, Tabouillot T, Russin TJ, Shanmugavelandy SS, Butler PJ, Eklund PC, Yun JK, Kester M, Adair JH (2008) Encapsulation of organic molecules in calcium phosphate nanocomposite particles for intracellular imaging and drug delivery. Nano Lett 8:4108–4115
Altinoglu EI, Russin TJ, Kaiser JM, Barth BM, Eklund PC, Kester M, Adair JH (2008) Near-infrared emitting fluorophore-doped calcium phosphate nanoparticles for in vivo imaging of human breast cancer. ACS Nano 2:2075–2084
Mokoena PP, Nagpure IM, Kumar V, Kroon RE, Olivier EJ, Neethling JH, Swart HC, Ntwaeaborwa OM (2014) Enhanced UVB emission and analysis of chemical states of Ca5(PO4)3OH:Gd3+, Pr3+ phosphor prepared by co-precipitation. J Phys Chem Solids 75:998–1003
Zhang C, Cheng Z, Yang P, Xu Z, Peng C, Li G, Lin J (2009) Architectures of strontium hydroxyapatite microspheres: solvothermal synthesis and luminescence properties. Langmuir 25:13591–13598
Zhang C, Li C, Huang S, Hou Z, Cheng Z, Yang P, Peng C, Lin J (2010) Self- activated luminescet and mesoporous strontium hydroxyapatite nanorods for drug delivery 31: 3374–3383
Naccache R, Rodriguez EM, Bogdan N, Sanz-Rodriguez F, Cruz MCI, Fuente AJ, Vetrone F, Jaque D, Sole JG, Capobianco JA (2012) High resolution fluorescence imaging of cancers using lanthanide ion-doped upconverting nanocrystals. Cancers 4:1067–1105
Cheng F, Sun K, ZhaoY LY, Xin Q, Sun X (2014) Synthesis and characterization of HA/YVO4: Yb3+, Er3+ up-conversion luminescent nano-rods. Ceram Int 40:1139–11334
Anuradha JJC, Gulati K, Ray A, Roy I (2014) Fluorophore-doped calcium phosphate nanoparticles for non-toxic biomedical applications. RSC Adv 4:40449–40455
Niemirowicz K, Markiewicz KH, Wilczewska AZ, Car H (2012) Magnetic nanoparticles as new diagnostic tools in medicine. Adv Med Sci 57:196–207
Yoo D, Lee J, Shin T, Cheon J (2011) Theranostic magnetic nanoparticles. Acc Chem Res 44:863–874
Laurent S, Forge D, Port M, Roch A, Robic C, Elst LV, Muller RN (2008) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterization, and biological applications. Chem Rev 108:2064–2110
Reddy H, Arias JL, Nicolas J, Couvreur (2012) Magnetic nanoparticles: design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem Rev 112:5818–5878
Singamaneni S, Bliznyuk VN, Binek C, Tsymbal EY (2011) Magnetic nanoparticles: recent advances in synthesis, self-assembly and applications. J Mater Chem 21:16819–16835
Gallo J, Long NJ, Aboagye EO (2013) Magnetic nanoparticles as contrast agents in the diagnosis and treatment of cancer. Chem Soc Rev 42:7816–7833
Gao J, Gu H, Xu B (2009) Multifunctional magnetic nanoparticles: design, synthesis, and biomedical applications. Acc Chem Res 42:1097–1107
Kaygili O, Dorozhkin SV, Ates T, Al-Ghamdi AA, Yakuphanoglu (2014) Dielectric properties of Fe doped hydroxyapatite prepare by sol–gel method. Ceram Int 40:9395–9402
Mercado DF, Magnacca G, Malandrino M, Rubert A, Montoneri E, Celi L, Prevot A, Gonzales MC (2014) Paramagnetic iron-doped hydroxyapatite nanoparticles with improved metal sorption properties. A bioorganic substrates-mediated synthesis. Appl Mater Interfaces 6:3937–3946
Liu X, Ma J, Yang J Visible-light-driven amorphous Fe(III)-substituted hydroxyapatite photocatalyst: Charecterization and photocatalytic activity. doi:10.1016/j.matlet.2014.09.018
Li Y, Ooi CP, Cheang PHN (2009) Synthesis and characterisation of neodymium (III) and gadolinium (III)- substituted hydroxyapatite as biomaterials. Int Appl Ceram Technol 4:501–512
Petchsang N, Pon-On W, Hodak JH, Tang IM (2009) Magnetic properties of Co-ferrite-doped hydroxyapatite nanoparticles having a core/shell structure. J Magn Magn Mater 321:1990–1995
Trandafir DL, Mirestean C, Turcu RVF, Frentiu B, Eniu D, Simon S (2014) Structural characterization of nanostructured hydroxyapatite-iron oxide composites. Ceram Int 40:11071–11078
Gopi D, Ansari MT, Shinyjoy E, Kavitha L (2012) Synthesis and spectroscopic charecterization of magnetic hydroxyapatite nanocomposite using ultrasonic irradiation. Spectrochim Acta A 87:245–250
Low HR, Phonthammachai N, Stewart GA, Bastow TJ, Ma LL, White TJ (2008) The crystal chemistry of ferric oxyhydroxyapatite. Inorg Chem 47:11774–11782
Li Y, Widodo J, Lim S, Ooi CP (2012) Synthesis and cytocompatibility of manganese (II) and iron (III) substituted hydroxyapatite nanaoparticles. J Mater Sci 47:754–763
Liu Y, Sun Y, Cao C, Yang Y, Wu Y, Ju D, Li F (2014) Long-term biodistribution in vivo and toxicity of radioactive/ magnetic hydroxyapatite nanaorods. Biomaterials 35:3348–3355
Tampieri A, Iafisco M, Sandri M, Panseri S, Cunha C, Sprio S, Savini E, Uhlarz M, Herrmannsdorfer T (2014) Magnetic bioinspired hybrid nanostructured collagen- hydroxyapatite scaffolds supporting cell proliferation and tuning regenerative process. Appl Mater Interfaces. doi:10.1021/am5050967
Panseri S, Cunha C, Dalessandro T, Sandri M, Giavaresi G, Marcacci M, Hung CT, Tampieri A (2012) Intrinsically superparamagnetic Fe-hydroxyapatite nanoparticles positively influences osteoblast-like cell behaviour. J Nanobiotechnol 10:32–41
Ruixue S, Kezheng C, Lei X (2013) Preparation and Characterization of Hydroxyapatite/ɤ-FE2O3 Hybrid Nanostructure. J Wuham Univ Technol Mater Sci Edu 215–219
Pon-On W, Meejoo S, Tang I (2008) Substitution of manganese and iron into hydroxyapatite: core/shell nanoparticle. Mater Res Bull 43:2137–2144
Iafisco M, Sandri M, Panseri S, Delgado-Lopez JM, Gomez-Morales J, Tampieri A (2013) Magnetic bioactive and biodegadable hollow Fe-doped hydroxyapatite coated poly(L-lactic) acid micro-nanospheres. Chem Mater 25:2610–2617
Tampieri A, DAlessandro T, Sandri M, Sprio S, Landi E, Bertinetti L, Panseri S, Pepponi G, Goettlicher J, Banobre-Lopez M, Rivas J (2012) Intrinsic magnetism and hyperthermia in bioactive Fe-doped hydroxyapatite. Acta Biomater 8:843–851
Kuda O, Pinchulk N, Iyanchenko L, Parkhomey O, Sych O, Leonowicz WR, Sowka E (2009) Effect of Fe3O4, Fe and Cu doping on magnetic properties and behaviour in physiological solution of biological hydroxyapatite/glass composite. J Mater Process Technol 209:1960–1964
Tseng C, Chang K, Yeh M, Yang K, Tang T, Lin F (2014) Development of a dual-functional Pt-Fe-HAP magnetic nanoparticles application for chemo-hyperthermia treatment of cancer. Ceram Int 40:5117–5127
Kanchana P, Lavanya N, Sekar C (2014) Development of amperometric L-tyrosine sensor based on Fe-doped hydroxyapatite nanoparticles. Mater Sci Eng C 35:85–91
Chandra VS, Baskar G, Suganthi RV, Elayaraja K, Joshy MIA, Beaula WS, Mythili R, Venkataraman G, Kalkura SN (2012) Blood compatibility of iron-doped nanosize hydroxyapatite and its drug release. Appl Mater Interfaces 4:1200–1210
Ashokan A, Menon D, Nair S, Koyakkutty M (2010) A molecular receptor targeted, hydroxyapatite nanocrystals based multi-modal contrast agent. Biomaterials 31:2606–2616
Liu Z, Wang Q, Yao S, Yang L, Yu S, Feng X, Li F (2014) Synthesis and characterization of Tb3+/Gd3+ dual-doped multifunctional hydroxyapatite nanaoparticle. Ceram Int 40:2613–2617
Li Z, Liu Z, Yin M, Yang X, Yuan Q, Ren JQX (2012) Aptamer-capped multifunctional mesoporous strontium hydroxyapatite nanovehicle for cancer-cell-responsive drug delivery and imaging. Biomacromolecules 13:4257–4263
Chen F, Huang P, Zhu Y, Wu J, Zhang C, Cui D (2011) The photoluminescence, drug delivery and imaging properties of multifunctional Eu3+/Gd3+ dual-doped hydroxyapatite nanorods. Biomaterials 32:9031–9039
Ashokan A, Gowd GS, Somasundaram VH, Bhupathi A, Peethambaran R, Unni AKK, Palaniswami SP, Nair SV, Koyakkutty M (2013) Multifunctional calcium phosphate nano-contrast agent for combined nuclear, magnetic and near-infrared in vivo imaging. Biomaterials 34:7143–7157
Chen F, Huang P, Zhu Y, Wu J, Cui D (2012) Multifunctional Eu3+/Gd3+ dual-doped calcium phosphate vesicle-like nanospheres for sustained drug release and imaging. Biomaterials 33:6447–6455
Liu M, Liu H, Sun S, Li X, Zhou Y, Hou Z, Lin J (2014) Multifunctional hydroxyapatite/Na(Y/Gd)F4:Yb3+, Er3+ composite fibres for drug delivery and dual model imaging. Langmuir 30:1176–1182
Syamchand SS, Priya S, Sony G (2015) Hydroxyapatite nanocrystals dually doped with fluorescent and paramagnetic labels for bimodal (luminomagnetic) cell imaging. Michrochim Acta 182:1213–1221
Yu MK, Park J, Jon S (2012) Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics 2:3–44
Veiseh O, Gunn JW, Zhang M (2010) Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv Drug Deliv Rev 62:284–304
Venkatasubbu GD, Ramasamy S, Avadhani GS, Ramakrishnan V, Kumar J (2013) Surface Modification and paclitaxel drug delivery of folic acid modified polyethylene glycol functionalized hydroxyapatite nanoparticles. Powder Technol 235:437–442
Yang Y, Liu C, Liang Y, Lin F, Wu K (2013) Hollow mesoporous hydroxyapatite nanoparticles (hmHANPs) with enhanced drug loading and pH-responsive release properties for intracellular drug delivery. J Mater Chem B 1:2447–2450
Wang S, Wang X, Xu H, Abe H, Tan Z, Zhao Y, Guo J, Naito M, Ichikawa H, Fukumori Y (2010) Towards sustained delivery of small molecular drugs using hydroxyapatite microspheres as the vehicles. Adv Powder Technol 21:268–272
Haifeng G, Zhiqiang Z, Feng Y, Guoping L, Zhiheng Z (2014) Preparation of magnetic, luminescent and mesoporous hydroxyapatite nanospindles with high specific surface area. Rare Metal Mater Eng 43:2647–2651
Vuong QL, Doorslaer SV, Bridot JL, Argante C, Alejandro G, Hermann R, Disch S, Mattea C, Stapf S, Gossum Y (2012) Paramagnetic nanoparticles as potential MRI contrast agents: characterization, NMR relaxation, simulations and theory. Magn Reson Mater Phys 25:467–478
Guo D, Xu K, Zhao X, Han Y (2005) Development of a strontium - containing hydroxyapatite bone cement. Biomaterials 26:4073–4083
Acknowledgments
The authors would like to acknowledge University Grant Commission (UGC) New Delhi, for providing financial assistance through the Teacher Fellowship under Faculty Improvement Programme (FIP) and the Head, Department of Chemistry, University of Kerala (Kariavattom Campus), Trivandrum.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Syamchand, S.S., Sony, G. Multifunctional hydroxyapatite nanoparticles for drug delivery and multimodal molecular imaging. Microchim Acta 182, 1567–1589 (2015). https://doi.org/10.1007/s00604-015-1504-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1504-x