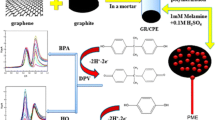
Abstract
An electrochemical method is presented for the determination of arsenic at subnanomolar levels. It is based on potentiometric stripping analysis (PSA) using a graphene paste electrode modified with the thiacrown 1,4,7-trithiacyclononane (TTCN) and gold nanoparticles (AuNPs). The electrode surface was characterized by means of cyclic voltammetry, electrochemical impedance spectroscopy, chronocoulometry and scanning electron microscopy. The modified electrode displays a 15-fold enhancement in the PSA signal (dt/dE) compared to a conventional graphene paste electrode. Under optimized conditions, the signal is proportional to the concentration of As(III) in the range from 25 pM to 34 nM (r2 = 0.9977), and the detection limit (SD/s) is as low as 8 pM. The modified electrode was successfully applied to the determination of total arsenic [i.e., As(III) and As(V)] in pharmaceutical formulations, human hair, sea water, fruits, vegetables, soil, and wine samples.

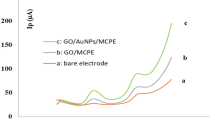
In the preconcentration step, arsenic forms stable intermetallic compounds with the gold substrate while allowing As to be reproducibly reoxidized during the stripping step. This, along with the complexing properties of the thiacrown TTCN towards As(III), results in a synergistic effect leading to a TTCN-AuNP-graphene paste electrode as a highly sensitive sensor for arsenic.




Similar content being viewed by others
References
Ivandini TA, Sato R, Makide Y, Fujishima A, Einaga Y (2006) Electrochemical detection of arsenic(III) using iridium-implanted boron-doped diamond electrodes. Anal Chem 78:6291
Johnson LR, Hiltbold AE (1969) Arsenic content of soil and crops following use of methane arsonate herbicides. Soil Sci Soc Am Proc 33:279
Radke B, Jewell L, Namieśnik J (2012) Analysis of arsenic species in environmental samples. Crit Rev Anal Chem 42:162
Song Y, Swain GM (2007) Development of a method for total inorganic arsenic analysis using anodic stripping voltammetry and a Au-coated, diamond thin-film electrode. Anal Chem 79:2412
Simm AO, Banks CE, Compton RG (2005) The electrochemical detection of As (III) at a silver electrode. Electroanalysis 17:1727
Dai X, Compton RG (2005) Gold nanoparticle modified electrodes show a reduced interference by Cu(II) in the detection of As(III) using anodic stripping voltammetry. Electroanalysis 17:1325
Sanghavi BJ, Wolfbeis OS, Hirsch T, Swami NS (2015) Nanomaterial-based electrochemical sensing of neurological drugs and neurotransmitters. Microchim Acta. doi:10.1007/s00604-014-1308-4
Sanghavi BJ, Mobin SM, Mathur P, Lahiri G, Srivastava AK (2013) Biomimetic sensor for certain catecholamines employing a copper(II) complex and silver nanoparticles modified glassy carbon paste electrode. Biosens Bioelectron 39:124
Sanghavi BJ, Srivastava AK (2013) Adsorptive stripping voltammetric determination of imipramine, trimipramine and desipramine employing titanium dioxide nanoparticles and an Amberlite XAD-2 modified glassy carbon paste electrode. Analyst 138:1395
Sanghavi BJ, Srivastava AK (2010) Simultaneous voltammetric determination of acetaminophen, aspirin and caffeine using an in situ surfactant-modified multiwalled carbon nanotube paste electrode. Electrochim Acta 55:8638
Gadhari NS, Sanghavi BJ, Srivastava AK (2011) Potentiometric stripping analysis of antimony based on carbon paste electrode modified with hexathia crown ether and rice husk. Anal Chim Acta 703:31
Gadhari NS, Sanghavi BJ, Karna SP, Srivastava AK (2010) Potentiometric stripping analysis of bismuth based on carbon paste electrode modified with cryptand [2.2.1] and multiwalled carbon nanotubes. Electrochim Acta 56:627
Gasnier A, Pedano ML, Rubianes MD, Rivas GA (2013) Graphene paste electrode: electrochemical behavior and analytical applications for the quantification of NADH. Sens Actuators B 176:921
Saber-Tehrani M, Pourhabib A, Husain SW, Arvand M (2012) Enhanced electrocatalytic reduction of oxadiargyl and its determination on 2-(4-(4-acetylphenyl)diazenyl) phenylamino)-ethanol modified graphene-paste electrode. Electroanalysis 24:2395
Shakibaian V, Parvin MH (2012) Determination of acetazolamide by graphene paste electrode. J Electroanal Chem 683:119
Hill NJ, Levason W, Reid G (2002) Arsenic(III) halide complexes with acyclic and macrocyclic thio- and seleno-ether Co-ligands: synthesis, spectroscopic and structural properties. Inorg Chem 41:2070
Atta NF, Galal A, Azab SM (2012) Novel sensor based on carbon paste/Nafion® modified with gold nanoparticles for the determination of glutathione. Anal Bioanal Chem 404:1661
Cubukcu M, Timur S, Anik U (2007) Examination of performance of glassy carbon paste electrode modified with gold nanoparticle and xanthine oxidase for xanthine and hypoxanthine detection. Talanta 74:434
Wimalasiri Y, Zou L (2013) Carbon nanotube/graphene composite for enhanced capacitive deionization performance. Carbon 59:464
Svancara I, Vytras K, Kalcher K, Walcarius A, Wang J (2008) Carbon paste electrodes in facts, numbers, and notes: a review on the occasion of the 50-years jubilee of carbon paste in electrochemistry and electroanalysis. Electroanalysis 21:7
Bose P, Sharma A (2002) Roll of iron in controlling speciation and mobilization of arsenic in subsurface environment. Water Res 36:4916
Chappel WR, Abernathy CO, Calderon L, Thomas DJ (2002) Arsenic Exposure and Health Effects V, Proceedings of the Fifth International Conference on Arsenic Exposure and Health Effects July 14–18 San Dieg California pp 351
Stopinski O (1976) Arsenic, national research council. National Academy of Sciences, US, p 318
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst A 32:751
Mester Z, Sturgeon RE (2001) Detection of volatile arsenic chloride species during hydride generation: a new prospectus. J Anal Atom Spectrom 16:470
Christie JH, Osteryoung RA, Anson FC (1967) Application of double potential-step chronocoulometry to the study of reactant adsorption theory. J Electroanal Chem Interfacial Electrochem 13:236
Miller JN, Miller JC (2010) Statistics and chemometrics for analytical chemistry pearson prentice hall 6th edn
Cui H, Yang W, Li X, Zhao H, Yuan Z (2012) An electrochemical sensor based on a magnetic Fe3O4 nanoparticles and gold nanoparticles modified electrode for sensitive determination of trace amounts of arsenic(III). Anal Methods 4:4176
Salaun P, Gibbon-Walsh KB, Alves GMS, Soares HMVM, van den Berg CMG (2012) Determination of arsenic and antimony in seawater by voltammetric and chronopotentiometric stripping using a vibrated gold microwire electrode. Anal Chim Acta 746:53
Dai X, Nekrassova O, Hyde ME, Compton RG (2004) Anodic stripping voltammetry of arsenic(III) using gold nanoparticle-modified electrodes. Anal Chem 76:5924
Lan Y, Luo H, Ren X, Wang Y, Liu Y (2012) Anodic stripping voltammetric determination of arsenic(III) using a glassy carbon electrode modified with gold-palladium bimetallic nanoparticles. Microchim Acta 178:153
Mardegan A, Scopece P, Lamberti F, Meneghetti M, Moretto LM, Ugo P (2012) Electroanalysis of trace inorganic arsenic with gold nanoelectrode ensembles. Electroanalysis 24:798
Alves GMS, Magalhaes JMCS, Salaun P, van den Berg CMG, Soares HMVM (2011) Simultaneous electrochemical determination of arsenic, copper, lead and mercury in unpolluted fresh waters using a vibrating gold microwire electrode. Anal Chim Acta 703:1
Rahman MR, Okajima T, Ohsaka T (2010) Selective detection of As(III) at the Au(111)-like polycrystalline gold electrode. Anal Chem 82:9169
Gibbon-Walsh K, Salaün P, van den Berg CMG (2010) Arsenic speciation in natural waters by cathodic stripping voltammetry. Anal Chim Acta 662:1
Acknowledgments
The funding for this work is partly by the University Grants Commission, New Delhi, India and partly by the US Army International Technology Center, Tokyo, Japan through contract number FA2386-12-1-4086.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 710 kb)
Rights and permissions
About this article
Cite this article
Sanghavi, B.J., Gadhari, N.S., Kalambate, P.K. et al. Potentiometric stripping analysis of arsenic using a graphene paste electrode modified with a thiacrown ether and gold nanoparticles. Microchim Acta 182, 1473–1481 (2015). https://doi.org/10.1007/s00604-015-1470-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1470-3