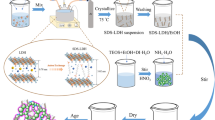
Abstract
We have synthesized, by a hydrothermal crystallization method, a layered double hydroxide of aluminum that is intercalated with the dodecyl sulfate anion. This nanocomposite is formed on a porous aluminum wire and can be used as a fiber coating for solid-phase microextraction. The nanocomposite has a flowerlike morphology and a high specific surface. The coating can be prepared easily, is mechanically stable, and exhibits relatively high thermal stability. It is capable of extracting phenolic compounds from water samples. Following thermal desorption, the phenols were quantified by GC-MS. The effects of extraction temperature, extraction time, sample ionic strength, stirring rate, pH, desorption temperature and desorption time were studied. Under optimal conditions, the repeatability for one fiber (for n = 5), expressed as the relative standard deviation, is between 3.9 and 7.6 %. The detection limits range from 0.2 to 4 pg mL−1. The method is simple, fast, and inexpensive. The fibers are thermally stable and yield better recoveries than conventional methods of analysis.

We have synthesized, by a hydrothermal crystallization method, a layered double hydroxide of aluminum that is intercalated with the dodecyl sulfate anion. This nanocomposite is formed on a porous aluminum wire and can be used as a fiber coating for solid-phase microextraction.





Similar content being viewed by others
References
Benzo M, Gilardoni G, Gandini C, Caccialanza G, Finzi PV, Vidari G, Abdo S, Layedra P (2007) Determination of the threshold odor concentration of main odorants in essential oils using gas chromatography–olfactometry incremental dilution technique. J Chromatogr A 1150:131–135
Gherman C, Culea M, Cozar O (2000) Comparative analysis of some active principles of herb plants by GC/MS. Talanta 53:253–262
Bertoli A, Pistelli L, Morelli I, Fraternale D, Giamperi L, Ricci D (2004) Volatile constituents of different parts (roots, stems and leaves) of Smyrnium olusatrum L. Flavour Fragr J 19:522–525
Gholivand MB, Shamsipur M, Shamizadeh M, Moradian R, Astinchap B (2014) Cobalt oxide nanoparticles as a novel high-efficiency fiber coating for solid phase microextraction of benzene, toluene, ethylbenzene and xylene from aqueous solutions. Anal Chim Acta 822:30–36
Abolghasemi MM, Karimi B, Yousefi V (2013) Periodic mesoporous organosilica with ionic liquid framework as a novel fiber coating for headspace solid-phase microextraction of polycyclic aromatic hydrocarbons. Anal Chim Acta 804:280–286
Gholivand MB, Piryaei M, Abolghasemi MM (2011) Anodized aluminum wire as a solid-phase microextraction fiber for rapid determination of volatile constituents in medicinal plant. Anal Chim Acta 701:1–5
Yu T, Lim B, Xia Y (2010) Aqueous-phase synthesis of single-crystal ceria nanosheets. Angew Chem Int Ed 49:4484–4487
Shibata T, Fukuda K, Ebina Y, Kogure T, Sasaki T (2008) One-nanometer-thick seed layer of unilamellar nanosheets promotes oriented growth of oxide crystal films. Adv Mater 20:231–235
Siril PF, Ramos L, Beaunier P, Archirel P, Etcheberry A, Remita H (2009) Synthesis of ultrathin hexagonal palladium nanosheets. Chem Mater 21:5170–5175
Hernan L, Morales J, Sánchez L, Santos J, Rodríguez-Castellón E, Martínez JL (2000) Kinetics of intercalation of lithium and sodium into lead sulfide-niobium sulfide ((PbS)1.14(NbS2)2). Chem Mater 12:3792–3797
Günes F, Shin HJ, Biswas C, Han GH, Kim ES, Chae SJ, Choi JY, Lee YH (2010) Layer-by-layer doping of few-layer graphene film. ACS Nano 8:4595–4600
Ma R, Sasaki T (2010) Nanosheets of oxides and hydroxides: ultimate 2D charge-bearing functional crystallites. Adv Mater 22:5082–5104
Zhang H, Xu ZP, Lu GQ, Smith SC (2009) Intercalation of sulfonate into layered double hydroxide: comparison of simulation with experiment. J Phys Chem C 113:559–563
Jaubertie C, Holgado MJ, SanRomán MS, Rives V (2006) Comparative study of the synthesis and properties of vanadate-exchanged layered double hydroxides. Chem Mater 18:3114–3121
Chen W, Feng L, Qu B (2004) Preparation of nanocomposites by exfoliation of ZnAl layered double hydroxides in nonpolar LLDPE solution. Chem Mater 16:368–370
Iyi N, Ebina Y, Sasaki T (2008) Water-swellable MgAl − LDH (layered double hydroxide) hybrids: synthesis, characterization, and film preparation. Langmuir 24:5591–5598
Xing LL, Yuan B, Hu SX, Zhang YD, Lu Y, Mai ZH, Li M (2008) Electrochemical synthesis of highly oriented layered zinc hydroxide with intercalated p-aminobenzoic acid. J Phys Chem C 112:3800–3809
Li B, He J (2008) Multiple effects of dodecanesulfonate in the crystal growth control and morphosynthesis of layered double hydroxides. J Phys Chem C 112:10909–10917
Romeo V, Gorrasi G, Vittoria V (2007) Encapsulation and exfoliation of inorganic lamellar fillers into polycaprolactone by electrospinning. Biomacromolecules 8:3147–3152
Xu ZP, Lu GQ (2005) Hydrothermal synthesis of layered double hydroxides (LDHs) from mixed MgO and Al2O3: LDH formation mechanism. Chem Mater 17:1055–1062
Lazaridis NK, Pandi TA, Matis KA (2004) Chromium(VI) removal from aqueous solutions by Mg − Al − CO3 hydrotalcite: sorption–desorption kinetic and equilibrium studies. Ind Eng Chem Res 43:2209–2215
Auxilio AR, Andrews PC, Junk PC, Spiccia L (2009) The adsorption behavior of C.I. Acid Blue 9 onto calcined Mg–Al layered double hydroxides. Dyes Pigm 81:103–112
Zhang P, Qian Gu X, Shi Hu ZP, Ruan X, Yang J, Frost RL (2012) Effective adsorption of sodium dodecylsulfate (SDS) by hydrocalumite (CaAl-LDH-Cl) induced by self-dissolution and re-precipitation mechanism. J Coll Int Sci 367:264–271
Hermonsín MC, Pavlovic I, UlibarrMA CJ (1997) Hydrotalcite as sorbent for trinitrophenol: sorption capacity and mechanism. Water Res 30:171–179
Wang B, Zhang H, Evans DG, Duan X (2005) Surface modification of layered double hydroxides and incorporation of hydrophobic organic compounds. Mat Chem Phys 92:190–196
You YW, Vance GF, Zhao HT (2001) Selenium adsorption on Mg–Al and Zn–Al layered double hydroxides. Appl Clay Sci 20:13–25
Zhao H, Nagy KL (2004) Dodecyl sulfate–hydrotalcite nanocomposites for trapping chlorinated organic pollutants in water. J Coll Int Sci 274:613–624
You YW, Zhao HT, Vance GF (2002) Surfactant-enhanced adsorption of organic compounds by layered double hydroxides. Colloids Surf A 205:161–165
You YW, Zhao HT, Vance GF (2002) Hybrid organic–inorganic derivatives of layered double hydroxides and dodecylbenzenesulfonate: preparation and adsorption characteristics. J Mater Chem 12:907–912
Dékány I, Berger F, Imrik K, Lagaly G (1997) Hydrophobic layered double hydroxides (LDHs): selective adsorbents for liquid mixtures. Colloid Polym Sci 275:681–688
Celis R, Koskinen WC, Cecchi AM, Bresnahan GA, Car-risoza MJ, Ulibarri M, Pavlovic I, Hermosín MC (1999) Sorption of the ionizable pesticide imazamox by organo‐clays and organohydrotalcites. J Environ Sci Health Part B 34:929–941
Celis R, Koskinen WC, Hermosín MC, Ulibarri MA, Cornejo J (2000) Triadimefon interactions with organoclays and organohydrotalcites. J Soil Sci Soc Am 64:36–43
Jakupca M, Dutta PK (1995) Growth of zinc sulfide thin films with the successive ionic layer adsorption and reaction method as studied by atomic force microscopy. Chem Mater 7:985–989
Dekany I, Haraszti T (1997) Synthesis and photocatalytic activity of TiO2-hectorite layer-by-layer thin films. Colloids Surf A 123:391–401
Aziz-Zanjani MO, Mehdinia A (2014) A review on procedures for the preparation of coatings for solid phase microextraction. Microchim Acta 181:1169–1190
Abdolmohammad-Zadeh H, Jouyban A, Amini R, Sadeghi G (2013) Nickel-aluminum layered double hydroxide as a nano-sorbent for the solid phase extraction of selenium, and its determination by continuous flow HG-AAS. Microchim Acta 180:619–626
Abdolmohammad-Zadeh H, Talleb Z (2012) Dispersive solid phase micro-extraction of dopamine from human serum using a nano-structured Ni-Al layered double hydroxide, and its direct determination by spectrofluorometry. Microchim Acta 179:25–32
Dutta PK, Robins R (1994) DS pyrene sorption in organic-layered double-metal hydroxides. Langmuir 10:1851–1856
Gholivand MB, Abolghasemi MM, Fattahpour P (2011) Polypyrrole/hexagonally ordered silica nanocomposite as a novel fiber coating for solid-phase microextraction. Anal Chim Acta 704:174–179
Gholivand MB, Abolghasemi MM, Fattahpour P (2012) Highly porous silica-polyaniline nanocomposite as a novel solid-phase microextraction fiber coating. J Sep Sci 35:101–106
Gholivand MB, Abolghasemi MM (2012) Inside needle capillary adsorption trap device for headspace solid-phase dynamic extraction based on polyaniline/hexagonally ordered silica nanocomposite. J Sep Sci 35:695–701
Abolghasemi MM, Yousefi V (2014) Three dimensionally honeycomb layered double hydroxides framework as a novel fiber coating for headspace solid-phase microextraction of phenolic compounds. J Chromatogr A 1345:9–16
Abolghasemi MM, Yousefi V, Hazizadeh B (2014) An inorganic–organic hybrid material based on ZnO nanoparticles anchored to a composite made from polythiophene and hexagonally ordered silica for use in solid-phase fiber microextraction of PAHs. Microchim Acta 181:639–645
Abolghasemi MM, Yousefi V, Rafiee E (2014) Keggin-type heteropoly compounds supported on montmorillonite clays offering strong option for efficient solid-phase microextraction coating. J Chromatogr A 1327:14–18
Abolghasemi MM, Yousefi V, Rafiee E (2014) Polyoxotungstate nanoclusters supported on silica as an efficient solid-phase microextraction fiber of polycyclic aromatic hydrocarbons. Microchim Acta 181:1807–1814
Boehm HP, Steinle J, Vieweger C (1977) [Zn2Cr(Oh)6]X·2H2O, New Layer Compounds Capable of Anion Exchange and Intracrystalline Swelling. Angew Chem Int Ed Engl 16:265–268
Drezdzon MA (1988) Synthesis of isopolymetalate-pillared hydrotalcite via organic-anion-pillared precursors. Inorg Chem 27:4628–4632
Bagheri H, Babanezhad E, Khalilian F (2008) A novel sol–gel-based amino-functionalized fiber for headspace solid-phase microextraction of phenol and chlorophenols from environmental samples. Anal Chim Acta 616:49–55
Ribeiro A, Neves MH, Almeida MF, Alves A, Santos L (2002) Direct determination of chlorophenols in landfill leachates by solid-phase micro-extraction-gas chromatography–mass spectrometry. J Chromatogr A 975:267–274
Alizadeh N, Zarabadipour H, Mohammadi A (2007) Headspace solid-phase microextraction using an electrochemically deposited dodecylsulfate-doped polypyrrole film to determine of phenolic compounds in water. Anal Chim Acta 605:159–165
Liu X, Ji Y, Zhang Y, Zhang H, Liu M (2007) Oxidized multiwalled carbon nanotubes as a novel solid-phase microextraction fiber for determination of phenols in aqueous samples. J Chromatogr A 1165:10–17
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 134 kb)
Rights and permissions
About this article
Cite this article
Abolghasemi, M.M., Yousefi, V. & Piryaei, M. Fabrication of a hierarchical dodecyl sulfate-layered double hydroxide nanocomposite on porous aluminum wire as an efficient coating for solid-phase microextraction of phenols. Microchim Acta 182, 1177–1186 (2015). https://doi.org/10.1007/s00604-014-1441-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1441-0