Abstract
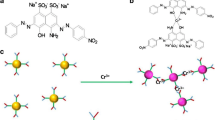
We present two colorimetric procedures for the determination of cyanuric acid, using silver nanoparticle-based (AgNPs) probes. The first is making use of melamine-modified AgNPs which bind to cyanuric acid through hydrogen bonding to form a large conjugate network that enhances the aggregation of AgNPs to produce an absorbance peak at 640 nm and a green coloration. In the second assay, melamine is directly added to the sample in order to form a stable complex with cyanuric acid. AgNPs are then added, resulting in the formation of an absorbance peaking at 525 nm and a color change from green (blank sample) to purple or orange-red as a function of cyanuric acid concentration. Matrix effects, that originate from the interaction of alkaline earth metals with the charged surface of the AgNPs, are mitigated through a matrix-matched calibration. In this manner, spectral transitions can be selectively attributed to the concentration of cyanuric acid, which can be even visually quantified at low mg L−1 levels with minimum sample pre-treatment and without sophisticated instrumentation.

Two colorimetric procedures for the determination of cyanuric acid, using silver nanoparticle-based (AgNPs) probes are presented. Matrix effects, which originate from the interaction of alkaline earth metals with the charged surface of the AgNPs, are mitigated through a matrix-matched calibration. In this manner, spectral transitions can be selectively attributed to the concentration of cyanuric acid, which can be visually quantified at low mg L−1 levels with minimum sample pre-treatment and no sophisticated instrumentation.



Similar content being viewed by others
References
Wojtowicz JA (1993a) “Cyanuric and Isocyanuric Acids.”, Encyclopedia of chemical technology, vol 7. John Wiley & Sons, Inc, New York, pp 834–851
Guenu S, Hennion MC (1994) On-line sample handling of water-soluble organic pollutants in aqueous samples using porous graphitic carbon. J Chromatogr A 665:243–251
Kowalsky L (1992) Certified Pool-SPA Operator; Ed.; National Swimming Pool Foundation, San Antonio, TX
Wojtowicz JA (2004) Effect of cyanuric acid on swimming pool maintenance. Journal of the Swimming Pool and Spa Industry 5:15–19
Downes CJ, Mitchell JW, Viotto ES, Eggers NJ (1984) Determination of cyanuric acid levels in swimming pool waters by U.V. absorbance, HPLC and melamine cyanurate precipitation. Water Res 18:277–280
Cantu R, Evans O, Kawahara FK, Wymer LJ, Dufour AP (2001) HPLC determination of cyanuric acid in swimming pool waters using phenyl and confirmatory porous graphitic carbon columns. Anal Chem 73:3358–3364
Cantu R, Evans O, Kawahara FK, Shoemaker JA, Dufour AP (2000) An HPLC method with UV detection, pH control, and reductive ascorbic acid for cyanuric acid analysis in water. Anal Chem 72:5820–5828
Pichon V, Chen L, Guenu S, Hennion MC (1995) Comparison of sorbents for the solid-phase extraction of the highly polar degradation products of atrazine (including ammeline, ammelide and cyanuric acid). J Chromatogr A 711:257–267
Latta D (1995) Interference in melamine–based determination of cyanuric acid concentration. Journal of the Swimming Pool and Spa Industry 1:37–39
Tsoumanis CM, Giokas DL, Vlessidis AG (2010) Monitoring and classification of wastewater quality using supervised pattern recognition techniques and deterministic resolution of molecular absorption spectra based on multiwavelength UV spectra deconvolution. Talanta 82:575–581
Briggle TV, Allen LM, Duncan RC, Pfaffenberger CD (1981) High performance liquid chromatographic determination of cyanuric acid in human urine and pool water. J Assoc Off Anal Chem 64:1222–1226
Yu C, Zhu L, Xiao J, Tang H, Guo G, Zeng Q, Wang X (2009) Ultrasonic extraction and determination of cyanuric acid in pet food. Food Control 20:205–208
Cantu R, Evans O, Magnuson ML (2001) Rapid analysis of cyanuric acid in swimming pool waters by high performance liquid chromatography using porous graphitic carbon. Chromatographia 53:454–456
Sun H, Qin X, Ge X, Wang L (2011) Effective separation and sensitive determination of cyanuric acid, melamine and cyromazine in environmental water by reversed phase high performance liquid chromatography. Environ Technol 32:317–323
Vaclavika L, Rosmusb J, Poppingc B, Hajslova J (2012) Rapid determination of melamine and cyanuric acid in milk powder using direct analysis in real time-time-of-flight mass spectrometry. J Chromatogr A 1217:4204–4211
Wang F, Hu S (2009) Electrochemical sensors based on metal and semiconductor nanoparticles. Microchim Acta 165:1–22
Ghosh SK, Pal T (2007) Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: from theory to applications. Chem Rev 107:4797–4862
Valdés MG, González ACV, Calzón JAG, Díaz-García ME (2009) Analytical nanotechnology for food analysis. Microchim Acta 166:1–19
Vilela D, González MC, Escarpa A (2012) Sensing colorimetric approaches based on gold and silver nanoparticles aggregation: chemical creativity behind the assay. A review. Anal Chim Acta 751:24–43
Lee JS, Lytton-Jean AKR, Hurst SJ, Mirkin CA (2007) Silver nanoparticle—oligonucleotide conjugates based on DNA with triple cyclic disulfide moieties. Nano Lett 7:2112–2115
Koutsoulis NP, Giokas DL, Vlessidis AG, Tsogas GZ (2010) Alkaline earth metal effect on the size and color transition of citrate-capped gold nanoparticles and analytical implications in periodate-luminol chemiluminescence. Anal Chim Acta 669:45–52
Polte J, Tuaev X, Wuithschick M, Fischer A, Thuenemann AF, Rademann K, Kraehnert R, Emmerling F (2012) Formation mechanism of colloidal silver nanoparticles: analogies and differences to the growth of gold nanoparticles. ACS Nano 6:5791–5802
Pinto VV, Ferreira MJ, Silva R, Santos HA, Silva F, Pereira CM (2010) Long time effect on the stability of silver nanoparticles in aqueous medium: effect of the synthesis and storage conditions. Colloids Surf A 364:19–25
Ma M, Bong D (2011) Determinants of cyanuric acid and melamine assembly in water. Langmuir 27:8841–8853
Ping H, Zhang M, Li H, Li S, Chen Q, Sun C, Zhang T (2012) Visual detection of melamine in raw milk by label-free silver nanoparticles. Food Control 23:191–197
El Badawy AM, Luxton TP, Silva RG, Scheckel KG, Suidan MT, Tolaymat TM (2010) Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ Sci Technol 44:1260–1266
Delay M, Dolt T, Woellhaf A, Sembritzki R, Frimmel FH (2011) Interactions and stability of silver nanoparticles in the aqueous phase: influence of natural organic matter (NOM) and ionic strength. J Chromatogr A 1218:4206–4212
Baalousha M, Nur Y, Römer I, Tejamaya M, Lead JR (2013) Effect of monovalent and divalent cations, anions and fulvic acid on aggregation of citrate-coated silver nanoparticles. Sci Total Environ 454–455:119–131
Li L, Li B, Cheng D, Mao L (2010) Visual detection of melamine in raw milk using gold nanoparticles as colorimetric probe. Food Chem 122:895–900
Yilmaz UT, Yazar Z (2010) Determination of cyanuric acid in swimming pool water and milk by differential pulse polarography. Clean-Soil, Air, Water 38:816–821
Miao H, Fan S, Wu YN, Zhang L, Zhou PP, Li JG, Chen HJ, Zhao YF (2009) Simultaneous determination of melamine, ammelide, ammeline, and cyanuric acid in milk and milk products by gas chromatography-tandem mass spectrometry. Biomed Environ Sci 22:87–94
Pan XQ, Wu PG, Yang DJ, Wang LY, Shen XH, Zhu CY (2013) Simultaneous determination of melamine and cyanuric acid in dairy products by mixed-mode solid phase extraction and GC-MS. Food Control 30:545–548
Desmarchelier A, Cuadra MG, Delatour T, Mottier P. Simultaneous quantitative determination of melamine and cyanuric acid in cow’s milk and milk-based infant formula by liquid chromatography-electrospray ionization tandem mass spectrometry. J Agric Food Chem 57:7186–7193.
Acknowledgments
This research has been co-financed by the European Union (European Social Fund – ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF) – Research Funding Program: THALES. Investing in knowledge society through the European Social Fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper was presented at the International Conference on Instrumental Methods of Analysis (IMA2013) in Thessaloniki, 15–19 September 2013.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 394 KB)
Rights and permissions
About this article
Cite this article
Kappi, F.A., Tsogas, G.Z., Giokas, D.L. et al. Colorimetric and visual read-out determination of cyanuric acid exploiting the interaction between melamine and silver nanoparticles. Microchim Acta 181, 623–629 (2014). https://doi.org/10.1007/s00604-014-1163-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1163-3