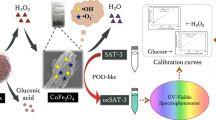
Abstract
Fe3O4 nanoparticles were deposited on sheets of graphene oxide (GO) by a precipitation method, and glucose oxidase (GOx) was then immobilized on this material to produce a GOx/Fe3O4/GO magnetic nanocomposite containing crosslinked enzyme clusters. The 3-component composite functions as a binary enzyme that was employed in a photometric method for the determination of glucose and hydrogen peroxide where the GOx/Fe3O4/GO nanoparticles cause the generation of H2O2 which, in turn, oxidize the substrate N,N-diethyl-p-phenylenediamine to form a purple product with an absorption maximum at 550 nm. The absorbance at 550 nm can be correlated to the concentration of glucose and/or hydrogen peroxide. Under optimized conditions, the calibration plot is linear in the 0.5 to 600 μM glucose concentration range, and the detection limit is 0.2 μM. The respective plot for H2O2 ranges from 0.1 to 10 μM, and the detection limit is 0.04 μM. The method was successfully applied to the determination of glucose in human serum samples. The GOx/Fe3O4/GO nanoparticles are reusable.

A one-step spectrophotometric method for the detection of glucose and/or H2O2 was developed by using GOx immobilized Fe3O4/GO MNPs as a bienzyme system and DPD as a substrate.







Similar content being viewed by others
References
Jin LH, Shang L, Guo SJ, Fang YX, Wen D, Wang L, Yin JY, Dong SJ (2011) Biomolecule-stabilized Au nanoclusters as a fluorescence probe for sensitive detection of glucose. Biosens Bioelectron 26:1965–1969
Guilbault GG, Brignac PJ, Zimmer M (1968) Homovanillic acid as a fluorometric substrate for oxidative enzymes. Analytical applications of the peroxidase, glucose oxidase, and xanthine oxidase systems. Anal Chem 40:190–196
Wei H, Wang EK (2008) Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal Chem 80:2250–2254
Chang Q, Zhu LH, Jiang GD, Tang HQ (2009) Sensitive fluorescent probes for determination of hydrogen peroxide and glucose based on enzyme immobilized magnetite/silica nanoparticles. Anal Bioanal Chem 395:2377–2385
Gao Y, Wang GN, Huang H, Hu JJ, Shah SM, Su XG (2011) Fluorometric method for the determination of hydrogen peroxide and glucose with Fe3O4 as catalyst. Talanta 85:1075–1080
Tang B, Zhang L, Xu KH (2006) FIA–near-infrared spectrofluorimetric trace determination of hydrogen peroxide using tricarchlorobocyanine dye (Cy.7.Cl) and horseradish peroxidase (HRP). Talanta 68:876–882
Chang Q, Deng KJ, Zhu LH, Jiang GD, Yu C, Tang HQ (2009) Determination of hydrogen peroxide with the aid of peroxidase-like Fe3O4 magnetic nanoparticles as the catalyst. Microchim Acta 165:299–305
Chen W, Chen J, Feng Y-B, Hong L, Chen Q-Y, Wu L-F, Lin X-H, Xia X-H (2012) Peroxidase-like activity of water-soluble cupric oxide nanoparticles and its analytical application for detection of hydrogen peroxide and glucose. Analyst (Cambridge, U K) 137:1706–1712
Song YJ, Qu KG, Zhao C, Ren JS, Qu XG (2010) Graphene oxide: intrinsic peroxidase catalytic activity and its application to glucose detection. Adv Mater 22:1–5
Li JH, Kuang DZ, Feng YL, Zhang FX, Liu MQ (2012) Glucose biosensor based on glucose oxidase immobilized on a nanofilm composed of mesoporous hydroxyapatite, titanium dioxide, and modified with multi-walled carbon nanotubes. Microchim Acta 176:73–80
Zhang JD, Oyama M (2004) A hydrogen peroxide sensor based on the peroxidase activity of hemoglobin immobilized on gold nanoparticles-modified ITO electrode. Electrochim Acta 50:85–90
Jiang GD, Tang HQ, Zhu LH, Zhang JD, Lu B (2009) Improving electrochemical properties of liquid phase deposited TiO2 thin films by doping sodium dodecylsulfonate and its application as bioelectrocatalytic sensor for hydrogen peroxide. Sens Actuator B Chem 138:607–612
Lan D, Li BX, Zhang ZJ (2008) Chemiluminescence flow biosensor for glucose based on gold nanoparticle-enhanced activities of glucose oxidase and horseradish peroxidase. Biosens Bioelectron 24:934–938
Chen LJ, Wang N, Wang XD, Ai SY (2013) Protein-directed in situ synthesis of platinum nanoparticles with superior peroxidase-like activity, and their use for photometric determination of hydrogen peroxide. Microchim Acta 180:1517–1522
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S, Yan X (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2:577–583
Wolfbeis OS, Oehme I, Papkovskaya N, Klimant I (2000) Sol–gel based glucose biosensors employing optical oxygen transducers, and a method for compensating for variable oxygen background. Biosens Bioelectron 15:69–76
Deng Y, Qi D, Deng C, Zhang X, Zhao D (2008) Superparamagnetic high-magnetization microspheres with an Fe3O4@SiO2 core and perpendicularly aligned mesoporous SiO2 shell for removal of microcystins. J Am Chem Soc 130:28–29
Veiseh O, Sun C, Gunn J, Kohler N, Gabikian P, Lee D, Bhattarai N, Ellenbogen R, Sze R, Hallahan A, Olson J, Zhang M (2005) Optical and MRI multifunctional nanoprobe for targeting gliomas. Nano Lett 5:1003–1008
Chen M-L, He Y-J, Chen X-W, Wang J-H (2012) Quantum dots conjugated with Fe3O4‑filled carbon nanotubes for cancer-targeted imaging and magnetically guided drug delivery. Langmuir 28:16469–16476
Wu S, Wang H, Tao S, Wang C, Zhang L, Liu Z, Meng C (2011) Magnetic loading of tyrosinase-Fe3O4/mesoporous silica core/shell microspheres for high sensitive electrochemical biosensing. Anal Chim Acta 686:81–86
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6:183–193
Pérez-López B, Arben M (2012) Carbon nanotubes and graphene in analytical sciences. Microchim Acta 179:1–16
Jiang GD, Lin ZF, Chen C, Zhu LH, Chang Q, Wang N, Wei W, Tang HQ (2011) TiO2 nanoparticles assembled on graphene oxide nanosheets with high photocatalytic activity for removal of pollutants. Carbon 49:2693–2701
Zhang F, Zheng B, Zhang JL, Huang XL, Liu H, Guo S, Zhang JY (2010) Horseradish peroxidase immobilized on graphene oxide: physical properties and applications in phenolic compound removal. J Phys Chem C 114:8469–8473
Hummers WS, Offeman RE (1958) Preparation of graphite oxide. J Am Chem Soc 80:1339–1339
Si YC, Samulski ET (2008) Exfoliated graphene separated by platinum nanoparticles. Chem Mater 20:6792–6797
Qian W, Chen ZQ, Cottingham S, Merrill WA, Swartz NA, Goforth AM, Clare TL, Jiao J (2012) Surfactant-free hybridization of transition metal oxide nanoparticles with conductive graphene for high-performance supercapacitor. Green Chem 14:371–377
Su J, Cao MH, Ren L, Hu CW (2011) Fe3O4-graphene nanocomposites with improved lithium storage and magnetism properties. J Phys Chem C 115:14469–14477
Lee J, Lee Y, Youn JK, Na HB, Yu T, Kim H, Lee S, Koo Y, Kwak JH, Park HG, Chang HN, Hwang M, Park J, Kim J, Hyeon T (2008) Simple synthesis of functionalized superparamagnetic magnetite/silica core/shell nanoparticles and their application as magnetically separable high-performance biocatalysts. Small 4:143–152
Acknowledgments
This project was supported by grants from the National Science Foundation of China (Nos. 21077037 and 21107143) and the Fundamental Research Funds for the Central Universities, South-Central University for Nationalities (No. CZQ11019).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 102 kb)
Rights and permissions
About this article
Cite this article
Chang, Q., Tang, H. Optical determination of glucose and hydrogen peroxide using a nanocomposite prepared from glucose oxidase and magnetite nanoparticles immobilized on graphene oxide. Microchim Acta 181, 527–534 (2014). https://doi.org/10.1007/s00604-013-1145-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-013-1145-x