Abstract
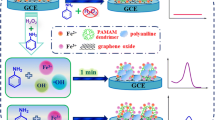
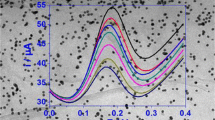
We report on a new electrochemical biosensing strategy for the sensitive detection of hydrogen peroxide (H2O2) in foodstuff samples. It is based on a gold electrode modified with layer of graphene patterned with a multilayer made from an organic–inorganic hybrid nanomaterial. Initially, a layer of thionine (Th) was assembled on the surface of the graphene nanosheets, and these were then cast on the surface of the electrode for the alternate assembly of gold nanoparticles and horseradish peroxidase. The large surface-to-volume ratio and high conductivity of the nanosheets provides a benign microenvironment for the construction of the biosensor. The use of such a multilayer not only shortens the electron transfer pathway of the active center of the enzyme due to the presence of gold nanoparticles, but also enhances the electrocatalytic efficiency of the biosensor toward the reduction of H2O2. The electrochemical characteristics of the biosensor were studied by cyclic voltammetry and chronoamperometry. The number of layers, the operating potential, and the pH of the supporting electrolyte were optimized. Linear response is obtained for the range from 0.5 μM to 1.8 mM of H2O2, the detection limit is 10 nM (at S/N = 3), and 95% of the steady-state current is reached within 2 s. The method was applied to sense H2O2 in spiked sterilized milk and correlated excellently with the permanganate titration method.

A new electrochemical biosensing strategy for sensitive detection of hydrogen peroxide in foodstuff was developed by using a gold electrode modified with a layer of graphene nanosheets patterned with a multilayer made from an organic–inorganic hybrid nanomaterial.







Similar content being viewed by others
References
Chang Q, Deng K, Zhu L, Jiang G, Yu C, Tang H (2009) Determination of hydrogen peroxide with the aid of peroxidased-like Fe3O4 magnetic nanoparticles as the catalyst. Microchim Acta 165:299
Young D, Mihaliak C, West S, Handelman K, Collins R, Phillips A, Robb C (2000) Determination of spinosad and its metabolites in food and environmental matrices. 3. Immunoassay methods. J Agric Food Chem 48:5146
Xu S, Zhang X, Wan T, Zhang C (2011) A third-generation hydrogen peroxide biosensor based on horseradish peroxidase cross-linked to multi-wall carbon nanotubes. Microchim Acta 172:199
Ping J, Ru S, Fan K, Wu J, Ying Y (2010) Copper oxide nanoparticles and ionic liquid modified carbon electrode for the non-enzymatic electrochemical sensing of hydrogen peroxide. Microchim Acta 171:117
Che X, Yuan R, Chai Y, Ma L, Li W, Li J (2009) Hydrogen peroxide sensor based on horseradish peroxidase immobilized on an electrode modified with DNA-L-cysteine-gold-platinum nanoparticles in polypyrrole film. Microchim Acta 167:159
Achatz D, Meier R, Fishcher L, Wolfbeis O (2011) Luminescent sensing of oxygen using a quenchable probe and upconverting nanoparticles. Angew Chem Int Ed 50:260
Pumer M, Ambrosi A, Bonanni A, Chng E, Poh H (2010) Graphene for electrochemical sensing and biosensing. TrAC Anal Chem 29:954
Willner I, Willner B, Tel-Vered R (2011) Electroanalytical applications of metallic nanoparticles and supramolecular nanostructurs. Electroanalysis 23:13
Nikolelis D, Hianik T, Nikoleli G (2010) Stabilized lipid films in electrochemical biosensors. Electroanalysis 22:2747
Lange U, Hirsch T, Mirsky V, Wolfbeis O (2011) Hydrogen sensor based on a graphene-palladium nanocomposite. Electrochim Acta. doi:10.1016/j.electacta.2010.10.078
Jia X, Campos-Delgado J, Terrones M, Meunier V, Dresselhaus M (2011) Graphene edges: a review of their fabrication and characterization. Nanoscale 3:86
Mas-Balleste R, Gomez-Navarro C, Gomez-Herrero J, Zamora F (2011) 2D materials: to graphene and beyond. Nanoscale 3:20
Brownson D, Banks C (2010) Graphene electrochemistry: an overview of potential applications. Analyst 135:2768
Pumera M (2010) Graphene-based nanomaterials and their electrochemistry. Chem Soc Rev 39:4146
Tosar J, Branas G, Laiz J (2010) Electrochemical DNA hybridization sensors applied to real and complex biological samples. Biosens Bioelectron 26:1205
Suryanarayanan V, Wu C, Ho K (2010) Molecularly imprinted electrochemical sensors. Electroanalysis 22:1795
Mantha S, Pedrosa V, Olsen E, Davis V, Simonian A (2010) Renewable nanocomposite layer-by-layer assembled catalytic interface for biosensing applications. Langmuir 26:19114
Nguyen D, Kim D, Kim K (2011) Controlled synthesis and biomolecular probe application of gold nanoparticles. Micron 42:207
Hayatt M (ed) (1989) Colloidal gold-principles, methods and applications. Academic, San Diego
Zhang Y, Tang Z, Fu X, Xu Y (2010) TiO2-graphene nanocomposites for gas-phase photocatalytic degradation of volatile aromatic pollutant: is TiO2-graphene truly different from other TiO2-carbon composite materials? ACS Nano 4:7303
Turkevich J, Stevenson P, Hillier J (1951) A study of the nucleation and growth process in the synthesis of colloidal gold. Discuss Faraday Soc 11:55
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci 241:20
Tang D, Tang J, Su B, Chen G (2011) Gold nanoparticles-decorated amine-terminated poly(amidoamine) dendrimer for sensitive electrochemical immunoassay of brevetoxins in food samples. Biosens Bioelectron 26:2090
Tang D, Yuan R, Chai Y (2008) Ultrasensitive electrochemical immunosensor for clinical immunoassay using thionine-doped magnetic gold nanospheres as labels and horseradish peroxidase as enhancer. Anal Chem 80:1582
Tang D, Yuan R, Chai Y (2006) Electrochemical immuno-bioanalysis for carcinoma antigen 125 based on thionine and gold nanoparticles modified carbon paste interface. Anal Chim Acta 564:158
Tang D, Ren J (2008) In situ amplified electrochemical immunoassay for carcinoembryonic antigen using horseradish peroxidase-encapsulated nanogold hollow microspheres as labels. Anal Chem 80:8064
Tang D, Yuan R, Chai Y (2006) Electron-transfer mediator microbiosensor fabrication based on immobilizing HRP-labeled Au colloids on gold electrode surface by 11-mercaptoundecanoic acid monolayer. Electroanalysis 18:259
He Y, Zheng J, Li K, Sheng Q, Qiao N (2010) A hydrogen peroxide biosensor based on room temperature ionic liquid functionalized graphene modified carbon ceramic electrode. Chin J Chem 28:2507
Zhang Y, Sun X, Zhu L, Shen H, Jia N (2011) Electrochemical sensing based on graphene/Prussian blue hybrid film modified electrode. Electrochim Acta 56:1239
Xu J, Liu C, Wu Z (2011) Direct electrochemistry and enhanced electrocatalytic activity of hemoglobin entrapped in graphene and ZnO nanospheres composite film. Microchim Acta. doi:10.1007/s00604-010-0515-x
Zhou Y, Liu S, Jiang H, Yang H, Chen H (2010) Direct electrochemistry and bioelectrocatalysis of microperoxidase-11 immobilized on chitosan-graphene nanocomposites. Electroanalysis 22:1323
Acknowledgement
The support by the National Natural Science Foundation of China (21075019, 20735002), the Research Fund for the Doctoral Program of Higher Education of China (20103514120003), the “973” National Basic Research Program of China (2010CB732403), and Program for Returned High-Level Overseas Chinese Scholars of Fujian Province (XRC-0929) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, Y., Zhang, B., Liu, B. et al. Sensitive detection of hydrogen peroxide in foodstuff using an organic–inorganic hybrid multilayer-functionalized graphene biosensing platform. Microchim Acta 174, 137–144 (2011). https://doi.org/10.1007/s00604-011-0608-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-011-0608-1