Abstract
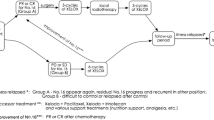
Dissection of the para-aortic lymph nodes (PAN) had once been enthusiastically explored at dedicated centers throughout Japan. Reflecting the results of a randomized trial, however, the current standard surgery for advanced resectable gastric cancer does not include systematic dissection of the PAN. Gastric cancer with PAN metastases, currently considered distant metastases, is classified as Stage IV, and according to the algorithm of the Japanese guidelines, is not indicated for surgery with curative intent. Historical data indicates, however, that a certain proportion of long-term survivors can be introduced among patients with PAN metastasis through D2 + PAN dissection. The Japan Clinical Oncology Group launched a series of phase II trials exploring a strategy employing neoadjuvant chemotherapy followed by D2 + PAN dissection for patients radiologically diagnosed to harbor metastases to the PAN. The campaign was successful, with 57 % of these patients surviving for 5 years after two cycles of neoadjuvant S-1/CDDP followed by surgery. This strategy is now the tentative standard, mentioned in the 4th version of the Japanese Gastric Cancer Treatment Guidelines as one of the current clinical questions, and could be replaced by a more powerful combination chemotherapy or treatment employing more or longer cycles of chemotherapy in the future. The relevance of the strategy consisting of neoadjuvant chemotherapy followed by D2 + PAN dissection and its fundamental difference from the concept of conversion therapy are discussed herein with reference to the literature.


Similar content being viewed by others
References
Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–46.
D’Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808–16.
Folprecht G, Gruenberger T, Bechstein W, Raab H-R, Weitz J, Lordick F, et al. Survival of patients with initially unresectable colorectal liver metastases treated with FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary concept (CELIM study). Ann Oncol. 2014;25:1018–25.
Kodera Y, Fujitani K, Fukushima N, Ito S, Muro K, Ohashi N, et al. Surgical resection of hepatic metastasis from gastric cancer: a review and new recommendation in the Japanese gastric cancer treatment guidelines. Gastric Cancer. 2014;17:206–12.
Sano T, Aiko T. New Japanese classification and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. 2011;14:97–100.
Takashima S, Kosaka T. Results and controversial issues regarding a para-aortic lymph node dissection for advanced gastric cancer. Surg Today. 2005;35:425–31.
Kunisaki C, Akiyama H, Nomura M, Matsuda G, Otsuka Y, Ono HA, et al. Comparison of surgical results of D2 versus D3 gastrectomy (para-aortic lymph node dissection) for advanced gastric carcinoma. A multi-institutional study. Ann Surg Oncol. 2006;13:659–67.
Fujimura T, Nakamura K, Oyama K, Funaki H, Fujita H, Kinami S, et al. Selective lymphadenectomy of para-aortic lymph nodes for advanced gastric cancer. Oncol Rep. 2009;22:509–14.
Roviello F, Pedrazzani C, Marrelli D, Di Leo A, Caruso S, Giacopuzzi S, et al. Super-extended (D3) lymphadenectomy in advanced gastric cancer. Eur J Surg Oncol. 2010;36:439–46.
Sawai K, Takahashi T, Suzuki H. New trends in surgery for gastric cancer in Japan. J Surg Oncol. 1994;56:221–6.
Kodera Y, Kinoshita M, Nakao A. Anatomical and lymphovascular basis of lymph node spread. In: Deguili M, editor. Management of gastric cancer: recent advances. Turin: Edizioni Minerva Medica; 2008. p. 149–60.
Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–62.
Kulig J, Popiela T, Kolodziejczyk P, Sierzega M, Szczepanik A. Standard D2 versus extended (D2+) lymphadenectomy for gastric cancer: an interim safety analysis of a multicenter, randomized, clinical trial. Am J Surg. 2007;193:10–5.
Yonemura Y, Wu CC, Fukushima N, et al. Randomized clinical trial of D2 and extended paraaortic lymphadenectomy in patients with gastric cancer. Int J Clin Oncol. 2008;13:132–7.
Yoshikawa T, Rino Y, Yukawa N, Oshima T, Tsuburaya A, Masuda M. Neoadjuvant chemotherapy for gastric cancer in Japan: a standing position by comparing with adjuvant chemotherapy. Surg Today. 2014;44:11–21.
Tokunaga M, Ohyama S, Hiki N, Fukunaga T, Aikou S, Yamaguchi T. Can superextended lymph node dissection be justified for gastric cancer with pathologically positive para-aortic lymph nodes? Ann Surg Oncol. 2010;17:2031–6.
Yoshida M, Ohtsu A, Boku N, Miyata Y, Shirao K, Shimada Y, et al. Survival and prognostic factors in patients with gastric cancers treated with chemotherapy in clinical oncology group (JCOG) study. Jpn J Clin Oncol. 2004;34:654–9.
Park IH, Kim SY, Kim YW, Ryu KW, Lee JH, Lee JS, et al. Clinical characteristics and treatment outcomes of gastric cancer patients with isolated para-aortic lymph node involvement. Cancer Chemother Pharmacol. 2011;67:127–36.
Hasegawa S, Yoshikawa T, Shirai J, Fujikawa H, Cho H, Doiuchi T, et al. A prospective validation study to diagnose serosal invasion and nodal metastases of gastric cancer by multidetector-row CT. Ann Surg Oncol. 2013;20:2016–22.
Marrelli D, Mazzei MA, Pedrazzani C, Martino MD, Vindigni C, Corso G, et al. High accuracy of multislices computed tomography (MSCT) for para-aortic lymph node metastases from gastric cancer. A prospective single-center study. Ann Surg Oncol. 2011;18:2265–72.
Yoshikawa T, Sasako M, Yamamoto S, Sano T, Imamura H, Fujitani K, et al. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg. 2009;96:1015–22.
Boku N, Ohtsu A, Shimada Y, Shirao K, Seki S, Saito H, et al. Phase II study of a combination of irinotecan and cisplatin against metastatic gastric cancer. J Clin Oncol. 1999;17:2915–21.
Tsuburaya A, Mizusawa J, Tanaka Y, Fukushima N, Nashimoto A, Sasako M, et al. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. 2014;101:653–60.
Koizumi W, Tanabe S, Saigenji K, Ohtsu A, Boku N, Nagashima F, et al. Phase I/II study of S-1 combined with cisplatin in patients with advanced gastric cancer. Br J Cancer. 2003;89:2207–12.
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for fiest-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21.
Katayama H, Ito S, Sano T, Takahari D, Mizusawa J, Boku N, et al. A phase II study of systemic chemotherapy with docetaxel, cisplatin and S-1/DCS) followed by surgery in gastric cancer patients with extensive lymph node metastasis: Japan Clinical Oncology Group study JCOG1002. Jpn J Clin Oncol. 2012;42:556–9.
Koizumi W, Nakayama N, Tanabe S, Sasaki T, Higuchi K, Nishimura K, et al. A multicenter phase II study of combined chemotherapy with docetaxel, cisplatin, and S-1 in patients with resectable or recurrent gastric cancer (KDOG 0601). Cancer Chemother Pharmacol. 2012;69:407–13.
Oyama K, Fushida S, Kinoshita J, Makino I, Nakamura K, Hayashi H, et al. Efficacy of pre-operative chemotherapy with docetaxel, cisplatin, and S-1 (DCS therapy) and curative resection for gastric cancer with pathologically positive para-aortic lymph nodes. J Surg Oncol. 2012;105:535–41.
Becker K, Mueller JD, Schumacher C, Ott K, Fink U, Busch T, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003;98:1521–30.
Kurokawa Y, Shibata T, Sasako M, Sano T, Tsuburaya A, Iwasaki Y, Fukuda H. Validation of response assessment criteria in neoadjuvant chemotherapy for gastric cancer (JCOG0507-A). Gastric Cancer. 2014;17:514–21.
Nakamura K, Kuwata T, Shimoda T, Mizusawa J, Katayama H, Kushima R, et al. Determination of the optimal cutoff percentage of residual tumors to define the pathological response rate for gastric cancer treated with preoperative therapy (JCOG1004-A). Gastric Cancer. 2014;. doi:10.1007/s10120-014-0401-z.
Feldman LD, Hortobagyi GN, Buzdar AU, Ames FC, Blumenschein GR. Pathologic assessment of response to induction chemotherapy in breast cancer. Cancer Res. 1986;46:2578–81.
Lowy AM, Mansfield PF, Leach SD, Pazdur R, Dumas P, Ajani JA. Response to noeadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann Surg. 1999;229:303–8.
Yoshikawa T, Tanabe K, Nishikawa K, Ito Y, Matsui T, Kimura Y, et al. Induction of a pathological complete response by four courses of neoadjuvant chemotherapy for gastric cancer: early results of the randomized phase II COMPASS trial. Ann Surg Oncol. 2014;21:213–9.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
Van Cutsem W, Bang Y-J, Feng-yi F, Xu JM, Lee K-W, Jiao S-C, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2014;. doi:10.1007/s10120-014-0402-y.
Matsumoto T, Sasako M, Mizusawa J, Hirota S, Ochiai A, Kushima R, et al. HER2 expression inlocally advanced gastric cancer with extensive lymph node (bulky N2 or paraaortic) metastasis (JCOG1005-A trial). Gastric Cancer. 2014;. doi:10.1007/s10120-014-0398-3.
Wang Y, Yu Y, Li W, Feng Y, Hou J, Ji Y, et al. A phase II trial of Xeloda and oxaliplatin (XELOX) neo-adjuvant chemotherapy followed by surgery for advanced gastric cancer patients with para-aortic lymph node metastasis. Cancer Chemother Pharmacol. 2014;73:1155–61.
Leone F, Artale S, Marino D, Cagnazzo C, Cascinu S, Pinto C, et al. Panitumumab in combination with infusional oxaliplatin and oral capecitabine for conversion therapy in patients with colon cancer and advanced liver metastasis. The MetaPan study. Cancer. 2013;119:3429–35.
Tanizawa Y, Terashima M, Tokunaga M, Bando E, Kawamura T, Sugisawa N, et al. Conversion therapy of stage IV gastric cancer. Jpn J Cancer Chemother (Gan To Kagaku Ryoho). 2012;39:2469–73 (In Japanese).
Conflict of interest
Yasuhiro Kodera has received research Grants from Taiho Pharmaceutical Company, Chugai Pharmaceutical Company, Yakult Honsha Co. Ltd, Bristol-Myers Squibb, Eli Lilly Japan KK, and Pfizer Japan, and received lecture fees from Taiho Pharmaceutical Company and Chugai Pharmaceutical Company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kodera, Y., Kobayashi, D., Tanaka, C. et al. Gastric adenocarcinoma with para-aortic lymph node metastasis: a borderline resectable cancer?. Surg Today 45, 1082–1090 (2015). https://doi.org/10.1007/s00595-014-1067-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-014-1067-1