Abstract
Purpose
Trehalose, naturally occurring disaccharide, has been reported to prevent postoperative abdominal adhesions in animal models. We investigated whether trehalose affects the function of human polymorphonuclear neutrophils (PMNs) in vitro to assess the feasibility of its clinical application as an anti-adhesive barrier.
Methods
Human PMNs were obtained from 17 healthy volunteers. Escherichia coli and Staphylococcus aureus were used for the bacterial infection model, whereas lipopolysaccharide (LPS) and interleukin (IL)-1β were used for inflammation induction model. The PMN phagocytosis rates of bacteria and apoptosis/necrosis were assessed on trehalose, maltose, and control media. Cytokines; namely, tumor necrosis factor-α, IL-1α, IL-1Ra, IL-6, and IL-8; and PMN-elastase were measured on each medium in both models.
Results
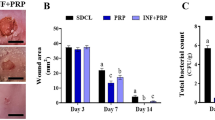
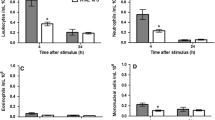
There were no significant differences in the phagocytosis rates, apoptosis/necrosis rates, or levels of all cytokines or PMN-elastase among the three media in the bacterial infection model. There were also no significant differences in the levels of all cytokines and PMN-elastase among the three media in the IL-1β inflammation induction model. PMN-elastase was lower in trehalose and maltose medium after LPS stimulation, at 3 and 24 h.
Conclusions
Our results suggest that trehalose does not affect the cellular function, cytokine production, or release of PMN-elastase of human PMNs in an in vitro bacterial infection model.



Similar content being viewed by others
Abbreviations
- TNF-α:
-
Tumor necrosis factor-α
- IL-1α:
-
Interleukin-1α
- IL-1Ra:
-
IL-1 receptor antagonist
- LPS:
-
Lipopolysaccharide
References
Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL. Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Dig Surg. 2001;18:260–73.
Ward BC, Panitch A. Abdominal adhesions: current and novel therapies. J Surg Res. 2011;165:91–111.
Wiseman DM. Adhesion related disease—adhesions related deaths. 2003. http://www.adhesion.org.
Wilson MS. Practicalities and costs of adhesions. Colorectal Dis. 2007;9:60–5.
Fazio VW, Cohen Z, Fleshman JW, van Goor H, Bauer JJ, Wolff BG, et al. Reduction in adhesive small-bowel obstruction by Seprafilm adhesion barrier after intestinal resection. Dis Colon Rectum. 2006;49:1–11.
Yamaoka T, Takahashi Y, Fujisato T, Lee CW, Tsuji T, Ohta T, et al. Novel adhesion prevention membrane based on a bioresorbable copoly(ester-ether) comprised of poly-l-lactide and pluronic: in vitro and in vivo evaluations. J Biomed Mater Res. 2001;54:470–9.
Diamond MP, Freeman ML. Clinical implications of postsurgical adhesions. Hum Reprod Update. 2001;7:567–76.
Kusunoki M, Ikeuchi H, Yanagi H, Noda M, Tonouchi H, Mohri Y, et al. Bioresorbable hyaluronate–carboxymethylcellulose membrane (Seprafilm) in surgery for rectal carcinoma: a prospective randomized clinical trial. Surg Today. 2005;35:940–5.
Mohri Y, Uchida K, Araki T, Inoue Y, Tonouchi H, Miki C, et al. Hyaluronic acid–carboxycellulose membrane (Seprafilm) reduces early postoperative small bowel obstruction in gastrointestinal surgery. Am Surg. 2005;71:861–3.
Inoue M, Uchida K, Miki C, Kusunoki M. Efficacy of Seprafilm for reducing reoperative risk in pediatric surgical patients undergoing abdominal surgery. J Pediatr Surg. 2005;40:1301–6.
Ouaïssi M, Gaujoux S, Veyrie N, Denève E, Brigand C, Castel B, et al. Post-operative adhesions after digestive surgery: their incidence and prevention: review of the literature. J Visc Surg. 2012;149:e104–14.
Kamel RM. Prevention of postoperative peritoneal adhesions. Eur J Obstet Gynecol Reprod Biol. 2010;150:111–8.
Elbein AD, Pan YT, Pastuszak I, Carroll D. New insights on trehalose: a multifunctional molecule. Glycobiology. 2003;13:17R–27R.
Chen Q, Haddad GG. Role of trehalose phosphate synthase and trehalose during hypoxia: from flies to mammals. J Exp Biol. 2004;207:3125–9.
Ohtake S, Wang YJ. Trehalose: current use and future applications. J Pharm Sci. 2011;100:2020–53.
Lee S, Miwa Y, Nishimura R, Chung UI, Suzuki S, Sasaki N. Effects of trehalose and sodium carboxymethyl cellulose on prevention of organ adhesion after laparotomy: a preliminary study. Jpn J Vet Anesth Surg. 2009;40:19–26.
Fujino H, Lee S, Suzuki S, Chung UI, Mochizuki M, Nishimura R, et al. Trehalose may prevent postsurgical adhesions in a rabbit model of hysterotomy. J Vet Med Sci. 2011;73:931–5.
Ohata A, Iwata K, Tamura N, Nojima H, Tanei S. Adhesion-reducing effects of trehalose formulation in the rabbit cecal and abdominal wall abrasion model. J Abdom Emerg Med. 2011;31:434 (Japanese).
Lim R, Morrill JM, Lynch RC, Reed KL, Gower AC, Leeman SE, et al. Practical limitations of bioresorbable membranes in the prevention of intra-abdominal adhesions. J Gastrointest Surg. 2009;13:35–41.
Uchida K, Urata H, Mohri Y, Inoue M, Miki C, Kusunoki M. Seprafilm does not aggravate intraperitoneal septic conditions or evoke systemic inflammatory response. Surg Today. 2005;35:1054–9.
Otake K, Uchida K, Yoshiyama S, Inoue M, Okita Y, Watanabe H, et al. Effects of a hyaluronate–carboxymethylcellulose membrane (Seprafilm) on human polymorphonuclear neutrophil functions. J Surg Res. 2008;149:243–9.
Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89.
Zysk G, Bejo L, Schneider-Wald BK, Nau R, Heinz H. Induction of necrosis and apoptosis of neutrophil granulocytes by Streptococcus pneumoniae. Clin Exp Immunol. 2000;122:61–6.
Turina M, Miller FN, McHugh PP, Cheadle WG, Polk HC Jr. Endotoxin inhibits apoptosis but induces primary necrosis in neutrophils. Inflammation. 2005;29:55–63.
Abbott PJ, Chen J. Trehalose. WHO food additive series 46. http://www.inchem.org/documents/jecfa/jecmono/v46je05.htm. Accessed December 22, 2010.
Esmann L, Idel C, Sarkar A, Hellberg L, Behnen M, Möller S, et al. Phagocytosis of apoptotic cells by neutrophil granulocytes: diminished proinflammatory neutrophil functions in the presence of apoptotic cells. J Immunol. 2010;184:391–400.
Ho SC, Lee KY, Chan YF, Kuo LW, Ito K, Adcock IM, et al. Neutrophil elastase represses IL-8/CXCL8 synthesis in human airway smooth muscle cells through induction of NF-kappa B repressing factor. J Immunol. 2009;183:411–20.
Acknowledgments
This study was funded by Otsuka Pharmaceutical Factory, Inc. (Tokushima, Japan). Trehalose and maltose were supplied by Otsuka Pharmaceutical Factory, Inc.
Conflict of interest
Masato Kusunoki consults to other clinical trials about the efficacy and safety of trehalose in the prevention of postsurgical adhesion formation conducted by Otsuka Pharmaceutical Factory, Inc. (Tokushima, Japan). Masato Kusunoki received a research grant for this study from Otsuka Pharmaceutical Factory, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanaka, K., Kawamura, M., Otake, K. et al. Trehalose does not affect the functions of human neutrophils in vitro. Surg Today 44, 332–339 (2014). https://doi.org/10.1007/s00595-013-0625-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-013-0625-2