Abstract
Aim
To investigate the frequency and spectrum of glucokinase (GCK) mutations in a cohort of adults from an island population having a high prevalence of diabetes mellitus (DM).
Methods
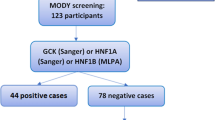
A single-centre cohort study was conducted, including 145 non-obese adults of Maltese-Caucasian ethnicity with impaired fasting glycaemia (IFG) or non-autoimmune diabetes diagnosed before the age of 40 years. Bidirectional sequencing of the GCK coding regions was performed. Genotype–phenotype associations and familial segregation were explored and the effects of missense variants on protein structure were evaluated using computational analysis.
Results
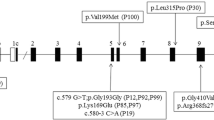
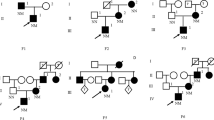
Three probands with pathogenic/likely pathogenic GCK variants in the heterozygous state having clinical features consistent with GCK-diabetes were detected. The missense variants have structurally destabilising effects on protein structure. GCK variant carriers exhibited a significantly lower body mass index and serum triglyceride levels when compared to GCK variant non-carriers.
Conclusions
The frequency of GCK-diabetes is approximately 2% in non-obese Maltese adults with diabetes or prediabetes. This study broadens the mutational spectrum of GCK and highlights clinical features that could be useful in discriminating GCK-DM from type 2 DM or prediabetes. It reinforces the need for increased molecular testing in young adults with diabetes having a suspected monogenic aetiology.

Similar content being viewed by others
Availability of data and material
The datasets generated during and/or analysed during the current study are available in the Mendeley Data repository, https://data.mendeley.com/datasets/w8mrxkvcwt/draft?a=7c4492d5-f81e-4ba1-808f-337d95a29c8b.
References
Firdous P, Nissar K, Ali S et al (2018) Genetic testing of maturity-onset diabetes of the young current status and future perspectives. Front Endocrinol 9:253. https://doi.org/10.3389/fendo.2018.00253
Ellard S, Bellanné-Chantelot C, Hattersley AT, Network EMGQ, (EMQN) MODY group, (2008) Best practice guidelines for the molecular genetic diagnosis of maturity-onset diabetes of the young. Diabetologia 51:546–553. https://doi.org/10.1007/s00125-008-0942-y
Velho G, Froguel P, Clement K et al (1992) Primary pancreatic beta-cell secretory defect caused by mutations in glucokinase gene in kindreds of maturity onset diabetes of the young. Lancet Lond Engl 340:444–448. https://doi.org/10.1016/0140-6736(92)91768-4
Gardner DS, Tai ES (2012) Clinical features and treatment of maturity onset diabetes of the young (MODY). Diabetes Metab Syndr Obes Targets Ther 5:101–108. https://doi.org/10.2147/DMSO.S23353
Osbak KK, Colclough K, Saint-Martin C et al (2009) Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum Mutat 30:1512–1526. https://doi.org/10.1002/humu.21110
Stride A, Shields B, Gill-Carey O et al (2014) Cross-sectional and longitudinal studies suggest pharmacological treatment used in patients with glucokinase mutations does not alter glycaemia. Diabetologia 57:54–56. https://doi.org/10.1007/s00125-013-3075-x
Pruhova S, Dusatkova P, Kraml PJ et al (2013) Chronic mild hyperglycemia in GCK-MODY patients does not increase carotid intima-media thickness. Int J Endocrinol 2013:718254. https://doi.org/10.1155/2013/718254
Dadwani RS, Skandari MR, GoodSmith MS et al (2020) Alternative type 2 diabetes screening tests may reduce the number of U.S. adults with undiagnosed diabetes. Diabet Med J Br Diabet Assoc 37:1935–1943. https://doi.org/10.1111/dme.14330
Nathan DM, Davidson MB, DeFronzo RA et al (2007) Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 30:753–759. https://doi.org/10.2337/dc07-9920
Gloyn AL, van de Bunt M, Stratton IM et al (2009) Prevalence of GCK mutations in individuals screened for fasting hyperglycaemia. Diabetologia 52:172–174. https://doi.org/10.1007/s00125-008-1188-4
Carmody D, Naylor RN, Bell CD et al (2016) GCK-MODY in the US national monogenic diabetes registry: frequently misdiagnosed and unnecessarily treated. Acta Diabetol 53:703–708. https://doi.org/10.1007/s00592-016-0859-8
Thanabalasingham G, Pal A, Selwood MP et al (2012) Systematic assessment of etiology in adults with a clinical diagnosis of young-onset type 2 diabetes is a successful strategy for identifying maturity-onset diabetes of the young. Diabetes Care 35:1206–1212. https://doi.org/10.2337/dc11-1243
Aloi C, Salina A, Minuto N et al (2017) Glucokinase mutations in pediatric patients with impaired fasting glucose. Acta Diabetol 54:913–923. https://doi.org/10.1007/s00592-017-1021-y
Shields BM, Hicks S, Shepherd MH et al (2010) Maturity-onset diabetes of the young (MODY): How many cases are we missing? Diabetologia 53:2504–2508. https://doi.org/10.1007/s00125-010-1799-4
Poon K-S, Tan KM-L, Koay ES-C, Sng A (2020) Genetic testing of GCK-MODY identifies a novel pathogenic variant in a Chinese boy with early onset hyperglycemia. Hum Genome Var 7:1–3. https://doi.org/10.1038/s41439-020-0096-0
Richards S, Aziz N, Bale S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–423. https://doi.org/10.1038/gim.2015.30
Tayoun ANA, Pesaran T, DiStefano MT et al (2018) Recommendations for interpreting the loss of function PVS1 ACMG/AMP variant criterion. Hum Mutat 39:1517–1524. https://doi.org/10.1002/humu.23626
Rodrigues CH, Pires DE, Ascher DB (2018) DynaMut: predicting the impact of mutations on protein conformation, flexibility and stability. Nucleic Acids Res 46:W350–W355. https://doi.org/10.1093/nar/gky300
Ittisoponpisan S, Islam SA, Khanna T et al (2019) Can predicted protein 3D structures provide reliable insights into whether missense variants are disease associated? J Mol Biol 431:2197–2212. https://doi.org/10.1016/j.jmb.2019.04.009
Biggerstaff BJ (2000) Comparing diagnostic tests: a simple graphic using likelihood ratios. Stat Med 19:649–663. https://doi.org/10.1002/(sici)1097-0258(20000315)19:5%3c649::aid-sim371%3e3.0.co;2-h
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (2001) Executive summary of the third report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 285:2486–2497. https://doi.org/10.1001/jama.285.19.2486
Flannick J, Johansson S, Njølstad PR (2016) Common and rare forms of diabetes mellitus: towards a continuum of diabetes subtypes. Nat Rev Endocrinol 12:394–406. https://doi.org/10.1038/nrendo.2016.50
Estalella I, Rica I, Nanclares GPD et al (2007) Mutations in GCK and HNF-1α explain the majority of cases with clinical diagnosis of MODY in Spain. Clin Endocrinol (Oxf) 67:538–546. https://doi.org/10.1111/j.1365-2265.2007.02921.x
Takeda J, Gidh-Jain M, Xu LZ et al (1993) Structure/function studies of human beta-cell glucokinase. Enzymatic properties of a sequence polymorphism, mutations associated with diabetes, and other site-directed mutants. J Biol Chem 268:15200–15204
Ma Y, Han X, Zhou X et al (2019) A new clinical screening strategy and prevalence estimation for glucokinase variant-induced diabetes in an adult Chinese population. Genet Med Off J Am Coll Med Genet 21:939–947. https://doi.org/10.1038/s41436-018-0282-3
Chakera AJ, Spyer G, Vincent N et al (2014) The 0.1% of the population with glucokinase monogenic diabetes can be recognized by clinical characteristics in pregnancy: the Atlantic diabetes in pregnancy cohort. Diabetes Care 37:1230–1236. https://doi.org/10.2337/dc13-2248
Steele AM, Wensley KJ, Ellard S et al (2013) Use of HbA1c in the identification of patients with hyperglycaemia caused by a glucokinase mutation: observational case control studies. PLoS ONE 8:e65326. https://doi.org/10.1371/journal.pone.0065326
Naylor RN, John PM, Winn AN et al (2014) Cost-effectiveness of MODY genetic testing: translating genomic advances into practical health applications. Diabetes Care 37:202–209. https://doi.org/10.2337/dc13-0410
Martin D, Bellanné-Chantelot C, Deschamps I et al (2008) Long-term follow-up of oral glucose tolerance test-derived glucose tolerance and insulin secretion and insulin sensitivity indexes in subjects with glucokinase mutations (MODY2). Diabetes Care 31:1321–1323. https://doi.org/10.2337/dc07-2017
Tinto N, Zagari A, Capuano M et al (2008) Glucokinase gene mutations: structural and genotype-phenotype analyses in MODY children from South Italy. PLoS ONE 3:e1870. https://doi.org/10.1371/journal.pone.0001870
Berger M, Mönks D, Schmidt H et al (2005) Are glucokinase mutations associated with low triglycerides? Clin Chem 51:791–793. https://doi.org/10.1373/clinchem.2004.045963
Ma M, Liu H, Yu J et al (2020) Triglyceride is independently correlated with insulin resistance and islet beta cell function: a study in population with different glucose and lipid metabolism states. Lipids Health Dis 19:121. https://doi.org/10.1186/s12944-020-01303-w
Iynedjian PB (2009) Molecular physiology of mammalian glucokinase. Cell Mol Life Sci CMLS 66:27–42. https://doi.org/10.1007/s00018-008-8322-9
Johnson SR, Ellis JJ, Leo PJ et al (2019) Comprehensive genetic screening: the prevalence of maturity-onset diabetes of the young gene variants in a population-based childhood diabetes cohort. Pediatr Diabetes 20:57–64. https://doi.org/10.1111/pedi.12766
Kleinberger JW, Copeland KC, Gandica RG et al (2018) Monogenic diabetes in overweight and obese youth diagnosed with type 2 diabetes: the TODAY clinical trial. Genet Med Off J Am Coll Med Genet 20:583–590. https://doi.org/10.1038/gim.2017.150
Raimondo A, Chakera AJ, Thomsen SK et al (2014) Phenotypic severity of homozygous GCK mutations causing neonatal or childhood-onset diabetes is primarily mediated through effects on protein stability. Hum Mol Genet 23:6432–6440. https://doi.org/10.1093/hmg/ddu360
Kesavan P, Wang L, Davis E et al (1997) Structural instability of mutant beta-cell glucokinase: implications for the molecular pathogenesis of maturity-onset diabetes of the young (type-2). Biochem J 322(Pt 1):57–63. https://doi.org/10.1042/bj3220057
Stride A, Vaxillaire M, Tuomi T et al (2002) The genetic abnormality in the beta cell determines the response to an oral glucose load. Diabetologia 45:427–435. https://doi.org/10.1007/s00125-001-0770-9
Sturis J, Kurland IJ, Byrne MM et al (1994) Compensation in pancreatic beta-cell function in subjects with glucokinase mutations. Diabetes 43:718–723. https://doi.org/10.2337/diab.43.5.718
Gragnoli C, Stanojevic V, Gorini A et al (2005) IPF-1/MODY4 gene missense mutation in an Italian family with type 2 and gestational diabetes. Metabolism 54:983–988. https://doi.org/10.1016/j.metabol.2005.01.037
Gonsorcikova L, Vaxillaire M, Pruhova S et al (2011) Familial mild hyperglycemia associated with a novel ABCC8-V84I mutation within three generations. Pediatr Diabetes 12:266–269. https://doi.org/10.1111/j.1399-5448.2010.00719.x
Dallali H, Pezzilli S, Hechmi M et al (2019) Genetic characterization of suspected MODY patients in Tunisia by targeted next-generation sequencing. Acta Diabetol 56:515–523. https://doi.org/10.1007/s00592-018-01283-5
Gašperíková D, Tribble ND, Staník J et al (2009) Identification of a novel β-cell glucokinase (GCK) promoter mutation (−71G>C) that modulates GCK gene expression through loss of allele-specific Sp1 binding causing mild fasting hyperglycemia in humans. Diabetes 58:1929–1935. https://doi.org/10.2337/db09-0070
Berberich AJ, Huot C, Cao H et al (2019) Copy number variation in GCK in patients with maturity-onset diabetes of the young. J Clin Endocrinol Metab 104:3428–3436. https://doi.org/10.1210/jc.2018-02574
Cuschieri S, Vassallo J, Calleja N et al (2016) The diabesity health economic crisis—the size of the crisis in a European island state following a cross-sectional study. Arch Public Health 74:52. https://doi.org/10.1186/s13690-016-0164-6
Goodrich JK, Singer-Berk M, Son R et al (2021) Determinants of penetrance and variable expressivity in monogenic metabolic conditions across 77,184 exomes. Nat Commun 12:3505. https://doi.org/10.1038/s41467-021-23556-4
Crouch DJM, Bodmer WF (2020) Polygenic inheritance, GWAS, polygenic risk scores, and the search for functional variants. Proc Natl Acad Sci 117:18924–18933. https://doi.org/10.1073/pnas.2005634117
Aykut A, Karaca E, Onay H et al (2018) Analysis of the GCK gene in 79 MODY type 2 patients: a multicenter Turkish study, mutation profile and description of twenty novel mutations. Gene 641:186–189. https://doi.org/10.1016/j.gene.2017.10.057
Funding
This work was supported by the University of Malta Research Excellence Grant.
Author information
Authors and Affiliations
Contributions
NPP and RC collected data. CAG, BV, and NPP performed the analyses. NPP and JV drafted the manuscript. All authors contributed to study conception and design and have read and approved the submitted manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
The study was approved by the institutional ethics review board of the University of Malta (71/2013 and 53/2009) and the study protocol was in compliance with the Declaration of Helsinki.
Consent to participate
All subjects gave written informed consent for their participation in the study and for genetic analysis.
Additional information
Managed by Massimo Porta.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pace, N.P., Grech, C.A., Vella, B. et al. Frequency and spectrum of glucokinase mutations in an adult Maltese population. Acta Diabetol 59, 339–348 (2022). https://doi.org/10.1007/s00592-021-01814-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-021-01814-7