Abstract
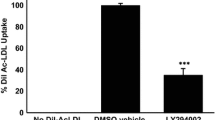
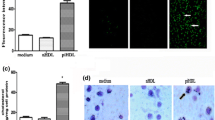
Studies show that dipeptidyl peptidase-4 (DPP-4) inhibitors may have an anti-atherosclerotic effect. Since foam cells are key components of atherosclerotic plaque, we studied the effect of DPP-4 inhibitors on foam cell formation. Foam cell formation was studied by treatment of THP-1 macrophages with oxidized low-density lipoprotein in the absence or presence of DPP-4 inhibitors (sitagliptin and NVPDPP728). The expression of scavenger receptors SRA, CD36 and LOX-1 was measured, and their role in foam cell formation in the presence of DPP-4 inhibitors was examined. In additional studies, role of protein kinase C and A in the effect of DPP-4 inhibitors was examined. Foam cell formation was markedly reduced by both DPP-4 inhibitors, as was the expression of CD36 and LOX-1 (CD36 ≫ LOX-1), but not SRA. Simultaneously, there was a reduction in phosphorylated PKC, but not PKA, content. Recovery of phosphorylated PKC following treatment of cells negated the effect of DPP-4 inhibitors on foam cell formation. Further, overexpression of CD36 or LOX-1 blocked the effect of DPP-4 inhibitors on foam cell formation. DPP-4 inhibitors repress foam cell formation through the inhibition of SRs CD36 and LOX-1, most likely via the inhibition of PKC activity. This study provides novel insights into the mechanism of inhibition of atherosclerosis by DPP-4 inhibitors.





Similar content being viewed by others
References
Ross R, Agius L (1992) The process of atherogenesis—cellular and molecular interaction: from experimental animal models to humans. Diabetologia 35:S34–S40
Mehta JL (2006) Oxidized or native low-density lipoprotein cholesterol: which is more important in atherogenesis? J Am Coll Cardiol 48:980–982
Crucet M, Wüst SJ, Spielmann P, Lüscher TF, Wenger RH, Matter CM (2013) Hypoxia enhances lipid uptake in macrophages: role of the scavenger receptors Lox1, SRA, and CD36. Atherosclerosis 229:110–117
Mehta JL, Sanada N, Hu CP, Chen J, Dandapat A, Sugawara F et al (2007) Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res 100:1634–1642
Goyal T, Mitra S, Khaidakov M, Wang X, Singla S, Ding Z et al (2012) Current concepts of the role of oxidized LDL receptors in atherosclerosis. Curr Atheroscler Rep 14:150–159
Mäkinen PI, Lappalainen JP, Heinonen SE, Leppänen P, Lähteenvuo MT, Aarnio JV et al (2010) Silencing of either SR-A or CD36 reduces atherosclerosis in hyperlipidaemic mice and reveals reciprocal upregulation of these receptors. Cardiovasc Res 88:530–538
Shiloh Y, Ziv Y (2013) The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol 14:197–210
Kong L, Shen X, Lin L, Leitges M, Rosario R, Zou YS et al (2013) PKCβ promotes vascular inflammation and acceleration of atherosclerosis in diabetic ApoE null mice. Arterioscler Thromb Vasc Biol 33:1779–1787
Ma L, Dong F, Denis M, Feng Y, Wang MD, Zha X (2011) Ht31, a protein kinase A anchoring inhibitor, induces robust cholesterol efflux and reverses macrophage foam cell formation through ATP-binding cassette transporter A1. J Biol Chem 286:3370–3378
Xiang J, Sun G, Mu Y, Liu H, Liu Y, Yang F et al (2011) Ritonavir stimulates foam cell formation by activating PKC. Chem Biol Interact 194:127–133
Namba M, Katsuno T, Kusunoki Y, Matsuo T, Miuchi M, Miyagawa J (2013) New strategy for the treatment of type 2 diabetes mellitus with incretin-based therapy. Clin Exp Nephrol 17:10–15
Dai Y, Dai D, Mercanti F, Ding Z, Wang X, Mehta JL (2013) Dipeptidyl peptidase-4 inhibitors in cardioprotection: a promising therapeutic approach. Acta Diabetol 50:827–835
Shah Z, Kampfrath T, Deiuliis JA, Zhong J, Pineda C, Ying Z et al (2011) Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation 124:2338–2349
Fadini GP, Avogaro A (2011) Cardiovascular effects of DPP-4 inhibition: beyond GLP-1. Vascul Pharmacol 55:10–16
Matsubara J, Sugiyama S, Akiyama E, Iwashita S, Kurokawa H, Ohba K et al (2013) Dipeptidyl peptidase-4 inhibitor, sitagliptin, improves endothelial dysfunction in association with its anti-inflammatory effects in patients with coronary artery disease and uncontrolled diabetes. Circ J 77:1337–1344
Krijnen PA, Hahn NE, Kholová I, Baylan U, Sipkens JA, van Alphen FP et al (2012) Loss of DPP4 activity is related to a prothrombogenic status of endothelial cells: implications for the coronary microvasculature of myocardial infarction patients. Basic Res Cardiol 107:233
Terasaki M, Nagashima M, Watanabe T, Nohtomi K, Mori Y, Miyazaki A et al (2012) Effects of PKF275-055, a dipeptidyl peptidase-4 inhibitor, on the development of atherosclerotic lesions in apolipoprotein E-null mice. Metabolism 61:974–977
Terasaki M, Nagashima M, Nohtomi K, Kohashi K, Tomoyasu M, Sinmura K et al (2013) Preventive effect of dipeptidyl peptidase-4 inhibitor on atherosclerosis is mainly attributable to incretin’s actions in nondiabetic and diabetic Apolipoprotein E-null mice. PLoS ONE 8:e70933
Park EK, Jung HS, Yang HI, Yoo MC, Kim C, Kim KS (2007) Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflamm Res 56:45–50
Voloshyna I, Modayil S, Littlefield MJ, Belilos E, Belostocki K, Bonetti L et al (2013) Plasma from rheumatoid arthritis patients promotes pro-atherogenic cholesterol transport gene expression in THP-1 human macrophages. Exp Biol Med (Maywood) 238:1192–1197
Jitprasertwong P, Jaedicke KM, Nile CJ, Preshaw PM, Taylor JJ (2013) Leptin enhances the secretion of interleukin (IL)-18, but not IL-1β, from human monocytes via activation of caspase-1. Cytokine. doi:10.1016/j.cyto.2013.10.008. [Epub ahead of print]
Chua S, Sheu JJ, Chen YL, Chang LT, Sun CK, Leu S et al (2013) Sitagliptin therapy enhances the number of circulating angiogenic cells and angiogenesis-evaluations in vitro and in the rat critical limb ischemia model. Cytotherapy 15:1148–1163
Dai Y, Mercanti F, Dai D, Wang X, Ding Z, Pothineni NV et al (2013) LOX-1, a bridge between GLP-1R and mitochondrial ROS generation in human vascular smooth muscle cells. Biochem Biophys Res Commun 437:62–66
Huang W, Ishii I, Zhang WY, Sonobe M, Kruth HS (2002) PMA activation of macrophages alters macrophage metabolism of aggregated LDL. J Lipid Res 43:1275–1282
Dai Y, Su W, Ding Z, Wang X, Mercanti F, Chen M et al (2013) Regulation of MSR-1 and CD36 in macrophages by LOX-1 mediated through PPAR-γ. Biochem Biophys Res Commun 431:496–500
Liu XY, Lu Q, Ouyang XP, Tang SL, Zhao GJ, Lv YC et al (2013) Apelin-13 increases expression of ATP-binding cassette transporter A1 via activating protein kinase C α signaling in THP-1 macrophage-derived foam cells. Atherosclerosis 226:398–407
Park KA, Byun HS, Won M, Yang KJ, Shin S, Piao L et al (2007) Sustained activation of protein kinase C downregulates nuclear factor-kappaB signaling by dissociation of IKK-gamma and Hsp90 complex in human colonic epithelial cells. Carcinogenesis 28:71–80
Michael DR, Ashlin TG, Davies CS, Gallagher H, Stoneman TW, Buckley ML et al (2013) Differential regulation of macropinocytosis in macrophages by cytokines: implications for foam cell formation and atherosclerosis. Cytokine 64:357–361
Kaplan M, Aviram M, Hayek T (2012) Oxidative stress and macrophage foam cell formation during diabetes mellitus-induced atherogenesis: role of insulin therapy. Pharmacol Ther 136:175–185
Li D, Liu L, Chen H, Sawamura T, Ranganathan S, Mehta JL (2003) LOX-1 mediates oxidized low-density lipoprotein-induced expression of matrix metalloproteinases in human coronary artery endothelial cells. Circulation 107:612–617
Lin CS, Lin FY, Ho LJ, Tsai CS, Cheng SM, Wu WL et al (2012) PKCδ signalling regulates SR-A and CD36 expression and foam cell formation. Cardiovasc Res 95:346–355
Antoniades C, Cunnington C, Antonopoulos A, Neville M, Margaritis M, Demosthenous M et al (2011) Induction of vascular GTP-cyclohydrolase I and endogenous tetrahydrobiopterin synthesis protect against inflammation-induced endothelial dysfunction in human atherosclerosis. Circulation 124:1860–1870
Acknowledgments
This study was supported by funds from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development, Washington, DC. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflict of interest
Yao Dai, Xianwei Wang, Zufeng Ding, Dongsheng Dai, Jawahar L. Mehta declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Massimo Federici.
Electronic supplementary material
Below is the link to the electronic supplementary material.
592_2013_541_MOESM1_ESM.tif
Figure supplement 1 (A) Dose-dependent effect of sitagliptin on foam cell formation. *P < 0.05 vs. 0 μg. (B) DPP-4 inhibitors repress the protein expression of CD36, LOX-1 and CD68 *P < 0.05 vs. control, †P < 0.05 vs. ox-LDL alone treatment group. Abbreviations same as in previous figures. (TIFF 240 kb)
592_2013_541_MOESM2_ESM.tif
Figure supplement 2 DAG reversed the inhibitory effects of DPP-4 inhibitors on CD36 and LOX-1 expression. (representative Western blotting, quantitative data on the right). *P < 0.05 vs. ox-LDL plus sitagliptin treatment, †P < 0.05 vs. ox-LDL plus NVPDPP728 treatment. (B) DPP-4 inhibitors repress the protein expression of IL-6. *P < 0.05 vs. control, †P < 0.05 vs. ox-LDL alone treatment group. (TIFF 84 kb)
592_2013_541_MOESM3_ESM.tif
Figure supplement 3 Confirmation of hCD36 and hLOX-1 cDNAs transfection efficiency. (A) CD36 and LOX-1 expression increased after transfection with hCD36 and hLOX-1 cDNA, respectively. *P < 0.05 vs. ox-LDL plus sitagliptin + empty vector; †P < 0.05 vs. ox-LDL plus NVPDPP728 + empty vector. B. IL-6, interleukin-6 expression increased after ox-LDL treatment and this increase was reversed by DPP-4 inhibitors. Abbreviations same as in previous figures. (TIFF 115 kb)
Rights and permissions
About this article
Cite this article
Dai, Y., Wang, X., Ding, Z. et al. DPP-4 inhibitors repress foam cell formation by inhibiting scavenger receptors through protein kinase C pathway. Acta Diabetol 51, 471–478 (2014). https://doi.org/10.1007/s00592-013-0541-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-013-0541-3