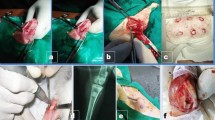
Abstract
Osteoarthritis (OA) is a major cause of suffering for millions of people. Investigating the disease directly on humans may be challenging. The aim of the present study is to investigate the advantages and limitations of the animal models currently used in OA research. The animal models are divided into induced and spontaneous. Induced models are further subdivided into surgical and chemical models, according to the procedure used to induce OA. Surgical induction of OA is the most commonly used procedure, which alters the exerted strain on the joint and/or alter load bearing leading to instability of the joint and induction of OA. Chemical models are generated by intra-articular injection of modifying factors or by systemically administering noxious agents, such as quinolones. Spontaneous models include naturally occurring and genetic models. Naturally occurring OA is described in certain species, while genetic models are developed by gene manipulation. Overall, there is no single animal model that is ideal for studying degenerative OA. However, in the present review, an attempt is made to clarify the most appropriate use of each model.


Similar content being viewed by others
References
Gore M, Tai KS, Sadosky A, Leslie D, Stacey BR (2011) Clinical comorbidities, treatment patterns, and direct medical costs of patients with osteoarthritis in usual care: a retrospective claims database analysis. J Med Econ 14(4):497–507
Vincent TL, Williams RO, Maciewicz R, Silman A, Garside P (2012) Mapping pathogenesis of arthritis through small animal models. Rheumatology (Oxford) 51(11):1931–1941
Abramson SB, Attur M (2009) Developments in the scientific understanding of osteoarthritis. Arthr Res Ther 11(3):227
Teeple E, Jay GD, Elsaid KA, Fleming BC (2013) Animal models of osteoarthritis: challenges of model selection and analysis. AAPS J [Epub ahead of print]
Bendele AM (2001) Animal models of osteoarthritis. J Musculoskelet Neuronal Interact 1(4):363–376
Frisbie DD, Cross MW, McIlwraith CW (2006) A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Vet Comp Orthop Traumatol 19(3):142–146
Giannoni P, Crovace A, Malpeli M, Maggi E, Arbico R, Cancedda R, Dozin B (2005) Species variability in the differentiation potential of in vitro-expanded articular chondrocytes restricts predictive studies on cartilage repair using animal models. Tissue Eng 11(1–2):237–248
Xerogeanes JW, Fox RJ, Takeda Y, Kim HS, Ishibashi Y, Carlin GJ, Woo SL (1998) A functional comparison of animal anterior cruciate ligament models to the human anterior cruciate ligament. Ann Biomed Eng 26(3):345–352
Brittberg M, Peterson L (1998) Introduction to an articular cartilage classification. ICRS Newsl 1:8
Little CB, Zaki S (2012) What constitutes an “animal model of osteoarthritis”—the need for consensus? Osteoarthr Cartil 20(4):261–267
Dontas IA (2007) The ethics of animal use in biomedical research. http://www.lmconference.com.au/papers/2007/dontas.html
Flecknel P (2009) Analgesia and post-operative care. In: Flecknel P (ed) Laboratory animal anaesthesia, 3rd edn. Academic Press, Amsterdam, pp 139–179
Mapp PI, Avery PS, McWilliams DF, Bowyer J, Day C, Moores S, Webster R, Walsh DA (2008) Angiogenesis in two animal models of osteoarthritis. Osteoarthr Cartil 16(1):61–69
Batiste DL, Kirkley A, Laverty S, Thain LM, Spouge AR, Holdsworth DW (2004) Ex vivo characterization of articular cartilage and bone lesions in a rabbit ACL transection model of osteoarthritis using MRI and micro-CT. Osteoarthr Cartil 12(12):986–996
Clements KM, Price JS, Chambers MG, Visco DM, Poole AR, Mason RM (2003) Gene deletion of either interleukin-1beta, interleukin-1beta-converting enzyme, inducible nitric oxide synthase, or stromelysin 1 accelerates the development of knee osteoarthritis in mice after surgical transection of the medial collateral ligament and partial medial meniscectomy. Arthr Rheum 48(12):3452–3463
Berjon JJ, Munuera L, Calvo M (1991) Degenerative lesions in the articular cartilage after meniscectomy: preliminary experimental study in dogs. J Trauma 31(3):342–350
Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS (1998) Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthr Rheum 41(4):687–693
Matsui Y, Iwasaki N, Kon S, Takahashi D, Morimoto J, Denhardt DT, Rittling S, Minami A, Uede T (2009) Accelerated development of aging-associated and instability-induced osteoarthritis in osteopontin-deficient mice. Arthr Rheum 60(8):2362–2371
Hayami T, Pickarski M, Zhuo Y, Wesolowski GA, Rodan GA, le Duong T (2006) Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone 38(2):234–243
Lindhorst E, Vail TP, Guilak F, Wang H, Setton LA, Vilim V, Kraus VB (2000) Longitudinal characterization of synovial fluid biomarkers in the canine meniscectomy model of osteoarthritis. J Orthop Res 18(2):269–280
Proffen BL, McElfresh M, Fleming BC, Murray MM (2012) A comparative anatomical study of the human knee and six animal species. Knee 19(4):493–499
Pond MJ, Nuki G (1973) Experimentally-induced osteoarthritis in the dog. Ann Rheum Dis 32(4):387–388
Yoshioka M, Coutts RD, Amiel D, Hacker SA (1996) Characterization of a model of osteoarthritis in the rabbit knee. Osteoarthr Cartil 4(2):87–98
Frost-Christensen LN, Mastbergen SC, Vianen ME, Hartog A, DeGroot J, Voorhout G, van Wees AM, Lafeber FP, Hazewinkel HA (2008) Degeneration, inflammation, regeneration, and pain/disability in dogs following destabilization or articular cartilage grooving of the stifle joint. Osteoarthr Cartil 16(11):1327–1335
Kamekura S, Hoshi K, Shimoaka T, Chung U, Chikuda H, Yamada T, Uchida M, Ogata N, Seichi A, Nakamura K, Kawaguchi H (2005) Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthr Cartil 13(7):632–641
Papaioannou NA, Triantafillopoulos IK, Khaldi L, Krallis N, Galanos A, Lyritis GP (2007) Effect of calcitonin in early and late stages of experimentally induced osteoarthritis. A histomorphometric study. Osteoarthr Cartil 15(4):386–395
Tochigi Y, Vaseenon T, Heiner AD, Fredericks DC, Martin JA, Rudert MJ, Hillis SL, Brown TD, McKinley TO (2011) Instability dependency of osteoarthritis development in a rabbit model of graded anterior cruciate ligament transection. J Bone Joint Surg Am 93(7):640–647
Sowers MR, McConnell D, Jannausch M, Buyuktur AG, Hochberg M, Jamadar DA (2006) Estradiol and its metabolites and their association with knee osteoarthritis. Arthr Rheum 54(8):2481–2487
Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, Dontas IA (2008) The laboratory rat as an animal model for osteoporosis research. Comp Med 58(5):424–430
Hoegh-Andersen P, Tanko LB, Andersen TL, Lundberg CV, Mo JA, Heegaard AM, Delaisse JM, Christgau S (2004) Ovariectomized rats as a model of postmenopausal osteoarthritis: validation and application. Arthr Res Ther 6(2):R169–R180
Sniekers YH, Weinans H, Bierma-Zeinstra SM, van Leeuwen JP, van Osch GJ (2008) Animal models for osteoarthritis: the effect of ovariectomy and estrogen treatment—a systematic approach. Osteoarthr Cartil 16(5):533–541
Ahern BJ, Parvizi J, Boston R, Schaer TP (2009) Preclinical animal models in single site cartilage defect testing: a systematic review. Osteoarthr Cartil 17(6):705–713
Mastbergen SC, Marijnissen AC, Vianen ME, van Roermund PM, Bijlsma JW, Lafeber FP (2006) The canine ‘groove’ model of osteoarthritis is more than simply the expression of surgically applied damage. Osteoarthr Cartil 14(1):39–46
Intema F, DeGroot J, Elshof B, Vianen ME, Yocum S, Zuurmond A, Mastbergen SC, Lafeber FP (2008) The canine bilateral groove model of osteoarthritis. J Orthop Res 26(11):1471–1477
Anraku Y, Mizuta H, Sei A, Kudo S, Nakamura E, Senba K, Hiraki Y (2009) Analyses of early events during chondrogenic repair in rat full-thickness articular cartilage defects. J Bone Miner Metab 27(3):272–286
Mrosek EH, Lahm A, Erggelet C, Uhl M, Kurz H, Eissner B, Schagemann JC (2006) Subchondral bone trauma causes cartilage matrix degeneration: an immunohistochemical analysis in a canine model. Osteoarthr Cartil 14(2):171–178
Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE, Juneau PL, Schrier DJ, Kilgore KS (2003) Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthr Cartil 11(11):821–830
Guingamp C, Gegout-Pottie P, Philippe L, Terlain B, Netter P, Gillet P (1997) Mono-iodoacetate-induced experimental osteoarthritis: a dose-response study of loss of mobility, morphology, and biochemistry. Arthr Rheum 40(9):1670–1679
Marker CL, Pomonis JD (2012) The monosodium iodoacetate model of osteoarthritis pain in the rat. Methods Mol Biol 851:239–248
Schuelert N, McDougall JJ (2009) Grading of monosodium iodoacetate-induced osteoarthritis reveals a concentration-dependent sensitization of nociceptors in the knee joint of the rat. Neurosci Lett 465(2):184–188
McDougall JJ, Pawlak M, Hanesch U, Schmidt RF (2000) Peripheral modulation of rat knee joint afferent mechanosensitivity by nociceptin/orphanin FQ. Neurosci Lett 288(2):123–126
Chandran P, Pai M, Blomme EA, Hsieh GC, Decker MW, Honore P (2009) Pharmacological modulation of movement-evoked pain in a rat model of osteoarthritis. Eur J Pharmacol 613(1–3):39–45
Miyauchi S, Machida A, Onaya J, Sakamoto T, Tokuyasu K, Iwata H (1993) Alterations of proteoglycan synthesis in rabbit articular cartilage induced by intra-articular injection of papain. Osteoarthr Cartil 1(4):253–262
van der Kraan PM, Vitters EL, van de Putte LB, van den Berg WB (1989) Development of osteoarthritic lesions in mice by “metabolic” and “mechanical” alterations in the knee joints. Am J Pathol 135(6):1001–1014
Schunke M, Tillmann B, Bruck M, Muller-Ruchholtz W (1988) Morphologic characteristics of developing osteoarthrotic lesions in the knee cartilage of STR/IN mice. Arthr Rheum 31(7):898–905
Sendzik J, Lode H, Stahlmann R (2009) Quinolone-induced arthropathy: an update focusing on new mechanistic and clinical data. Int J Antimicrob Agents 33(3):194–200
Bendele AM, Hulman JF (1988) Spontaneous cartilage degeneration in guinea pigs. Arthr Rheum 31(4):561–565
McDougall JJ, Andruski B, Schuelert N, Hallgrimsson B, Matyas JR (2009) Unravelling the relationship between age, nociception and joint destruction in naturally occurring osteoarthritis of Dunkin Hartley guinea pigs. Pain 141(3):222–232
Liu W, Burton-Wurster N, Glant TT, Tashman S, Sumner DR, Kamath RV, Lust G, Kimura JH, Cs-Szabo G (2003) Spontaneous and experimental osteoarthritis in dog: similarities and differences in proteoglycan levels. J Orthop Res 21(4):730–737
Carlson CS, Loeser RF, Purser CB, Gardin JF, Jerome CP (1996) Osteoarthritis in cynomolgus macaques. III: effects of age, gender, and subchondral bone thickness on the severity of disease. J Bone Miner Res 11(9):1209–1217
Regan E, Flannelly J, Bowler R, Tran K, Nicks M, Carbone BD, Glueck D, Heijnen H, Mason R, Crapo J (2005) Extracellular superoxide dismutase and oxidant damage in osteoarthritis. Arthr Rheum 52(11):3479–3491
Saamanen AK, Salminen HJ, Dean PB, De Crombrugghe B, Vuorio EI, Metsaranta MP (2000) Osteoarthritis-like lesions in transgenic mice harboring a small deletion mutation in type II collagen gene. Osteoarthr Cartil 8(4):248–257
Morko J, Kiviranta R, Joronen K, Saamanen AM, Vuorio E, Salminen-Mankonen H (2005) Spontaneous development of synovitis and cartilage degeneration in transgenic mice overexpressing cathepsin K. Arthr Rheum 52(12):3713–3717
Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, Turner J, Wu W, Billinghurst C, Meijers T, Poole AR, Babij P, DeGennaro LJ (2001) Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest 107(1):35–44
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lampropoulou-Adamidou, K., Lelovas, P., Karadimas, E.V. et al. Useful animal models for the research of osteoarthritis. Eur J Orthop Surg Traumatol 24, 263–271 (2014). https://doi.org/10.1007/s00590-013-1205-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-013-1205-2