Abstract
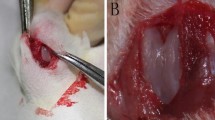
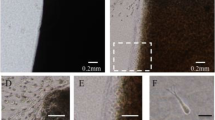
In this study we investigated the cellular events that occur during the onset of chondrogenic differentiation during the repair of full-thickness defects of articular cartilage. The V-shaped full-thickness cartilage defects (width 0.7 or 1.5 mm; depth 0.8 mm; length 4 mm) were created in the femoral patellar groove of rats using a custom-built twin-blade device. The time course of the repair response in these cartilage defects was examined using a semi-quantitative histological grading scale. Cartilaginous repair responses failed to occur in the larger 1.5 mm defects, which was covered only by fibrous scar tissue. In contrast, hyaline-like articular cartilage was regenerated concomitantly with the repair of the subchondral bone by 4 weeks in smaller 0.7 mm width defects. Cells in the reparative regions were then characterized by immunohistochemistry and in situ hybridization. Undifferentiated mesenchymal cells migrate into the defects and fill the cavities within 4 days of their creation. The expression of PCNA, N-cadherin, and PTH/PTHrP receptors was induced in cells at the center of the defects, where type II collagen-positive polygonal-shaped cells also begin to appear at day 7. Marrow-derived mesenchymal cells acquire higher levels of proliferative activity in induced cartilage cavities after their initial migration and filling of the smaller 0.7 mm defects. During the regenerative repair of articular cartilage in the rat, there is a distinctive step that appears to be analogous to the precartilaginous condensation that is pivotal during chondrogenesis in development.








Similar content being viewed by others
References
Buckwalter J, Rosenberg L, Coutts R, Hunziker E, Reddi AH, Mow V (1988) Articular cartilage: injury and repair of the musculoskeletal soft tissues. In: Woo SL-Y, Buckwalter JA (eds) American Academy of Orthopaedic Surgeons. Park Ridge, pp 465–482
Hunziker EB, Rosenberg LC (1996) Repair of partial-thickness defects in articular cartilage: cell recruitment from the synovial membrane. J Bone Joint Surg Am 78:721–733
Convery F, Akeson W, Koewn G (1972) The repair of osteochondral defects: an experimental study in horses. Clin Orthop 82:253–262
Kim HK, Moran ME, Salter RB (1991) The potential for regeneration of articular cartilage in defects created by chondral shaving and subchondral abrasion. An experimental investigation in rabbits. J Bone Joint Surg Am 73:1301–1315
Shapiro F, Koide S, Glimcher MJ (1993) Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am 75:532–553
DePalma AF, McKeever CD, Subin DK (1966) Process of repair of articular cartilage demonstrated by histology and autoradiography with tritiated thymidine. Clin Orthop 48:229–242
Solchaga LA, Goldberg VM, Caplan AI (2001) Cartilage regeneration using principles of tissue engineering. Clin Orthop Relat Res 391 Suppl:S161–S170
Otsuka Y, Mizuta H, Takagi K, Iyama K, Yoshitake Y, Nishikawa K, Suzuki F, Hiraki Y (1997) Requirement of fibroblast growth factor signaling for regeneration of epiphyseal morphology in rabbit full-thickness defects of articular cartilage. Dev Growth Differ 39:143–156
Kudo S, Mizuta H, Otsuka Y, Takagi K, Hiraki Y (2000) Inhibition of chondrogenesis by parathyroid hormone in vivo during repair of full-thickness defects of articular cartilage. J Bone Miner Res 15:253–260
Chuma H, Mizuta H, Kudo S, Takagi K, Hiraki Y (2004) One day exposure to FGF-2 was sufficient for the regenerative repair of full-thickness defects of articular cartilage in rabbits. Osteoarthritis Cartilage 12:834–842
Mizuta H, Kudo S, Nakamura E, Otsuka Y, Takagi K, Hiraki Y (2004) Active proliferation of mesenchymal cells prior to the chondrogenic repair response in rabbit full-thickness defects of articular cartilage. Osteoarthritis Cartilage 12:586–596
Noguchi T, Oka M, Fujino M, Neo M, Yamamuro T (1994) Repair of osteochondral defects with grafts of cultured chondrocytes. Comparison of allografts and isografts. Clin Orthop Relat Res 312:251–258
Grundnes O, Reikeras O (1995) Effects of function and weight-bearing on the healing of full-thickness cartilage defects in rats. Scand J Med Sci Sports 5:297–301
Nishimori M, Deie M, Kanaya A, Exham H, Adachi N, Ochi M (2006) Repair of chronic osteochondral defects in rat. A bone marrow-stimulating procedure enhanced by cultured allogenic bone marrow mesenchymal stroma cells. J Bone Joint Surg Br 88(9):1236–1244
Yoshioka M, Kubo T, Coutts RD, Hirasawa Y (1998) Differences in the repair process of longitudinal and transverse injuries of cartilage in the rat knee. Osteoarthritis Cartilage 6:66–75
Anraku Y, Mizuta H, Sei A, Kudo S, Nakamura E, Senba K, Takagi K, Hiraki Y (2008) The chondrogenic repair response of undifferentiated mesenchymal cells in rat full-thickness articular cartilage defects. Osteoarthritis Cartilage 16(8):961–964
Pineda S, Pollack A, Stevenson S, Goldberg V, Caplan A (1992) A semiquantitative scale for histologic grading of articular cartilage repair. Acta Anat Basel 143:335–340
Mizuta H, Kudo S, Nakamura E, Takagi K, Hiraki Y (2006) Expression of the PTH/PTHrP receptor in chondrogenic cells during the repair of full-thickness defects of articular cartilage. Osteoarthritis Cartilage 14:944–952
Fujii T, Ueno T, Kagawa T, Sugawara T, Yamamoto T (2005) Immunohistochemical analysis of Sox-9 expression in periosteum of tibia and calvaria after surgical release of periosteum. Acta Histochem 106(6):427–437
Tang GH, Rabie AB (2005) Runx2 regulates endochondral ossification in condyle during mandibular advancement. J Dent Res 84(2):166–171
Ferrari SL, Traianedes K, Thorne M, Lafage-Proust MH, Genever P, Cecchini MG, Behar V, Bisello A, Chorev M, Rosenblatt M, Suva LJ (2000) A role for N-cadherin in the development of the differentiated osteoblastic phenotype. J Bone Miner Res 15:198–208
Schulze C, Firth JA (1993) Immunohistochemical localization of adherens junction components in blood-brain barrier microvessels of the rat. J Cell Sci 104(Pt 3):773–782
Lagunowich LA, Donoso LA, Grunwald GB (1990) Identification of mammalian and invertebrate analogues of the avian calcium-dependent cell adhesion protein N-cadherin with synthetic-peptide directed antibodies against a conserved cytoplasmic domain. Biochem Biophys Res Commun 172:313–320
Amizuka N, Lee HS, Kwan MY, Arazani A, Warshawsky H, Hendy GN, Ozawa H, White JH, Goltzman D (1997) Cell-specific expression of the parathyroid hormone (PTH)/PTH-related peptide receptor gene in kidney from kidney-specific and ubiquitous promoters. Endocrinology 138:469–481
van der Eerden BC, Karperien M, Gevers EF, Lowik CW, Wit JM (2000) Expression of Indian hedgehog, parathyroid hormone-related protein, and their receptors in the postnatal growth plate of the rat: evidence for a locally acting growth restraining feedback loop after birth. J Bone Miner Res 15:1045–1055
Urena P, Kong XF, Abou-Samra AB, Juppner H, Kronenberg HM, Potts JT Jr, Segre GV (1993) Parathyroid hormone (PTH)/PTH-related peptide receptor messenger ribonucleic acids are widely distributed in rat tissues. Endocrinology 133:617–623
Scheven BA, Hamilton NJ, Farquharson C, Rucklidge GJ, Robins SP (1988) Immunohistochemical localization of native and denatured collagen types I and II in fetal and adult rat long bones. Bone 9:407–414
Alvarez J, Balbin M, Santos F, Fernandez M, Ferrando S, Lopez JM (2000) Different bone growth rates are associated with changes in the expression pattern of types II and X collagens and collagenase 3 in proximal growth plates of the rat tibia. J Bone Miner Res 15:82–94
George M, Chepenik KP, Schneiderman MH (1983) Proliferation of cells undergoing chondrogenesis in vitro. Differentiation 24:245–249
Shukunami C, Shigeno C, Atsumi T, Ishizeki K, Suzuki F, Hiraki Y (1996) Chondrogenic differentiation of clonal mouse embryonic cell line ATDC5 in vitro: differentiation-dependent gene expression of parathyroid hormone (PTH)/PTH-related peptide receptor. J Cell Biol 133:457–468
Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, Karperien M, Defize LH, Ho C, Mulligan RC, Abou-Samra AB, Juppner H, Segre GV, Kronenberg HM (1996) PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science 273:663–666
Chung UI, Lanske B, Lee K, Li E, Kronenberg H (1998) The parathyroid hormone/parathyroid hormone-related peptide receptor coordinates endochondral bone development by directly controlling chondrocyte differentiation. Proc Natl Acad Sci USA 95:13030–13035
Huang W, Chung UI, Kronenberg HM, de Crombrugghe B (2001) The chondrogenic transcription factor Sox9 is a target of signaling by the parathyroid hormone-related peptide in the growth plate of endochondral bones. Proc Natl Acad Sci USA 98:160–165
Shukunami C, Ohta Y, Sakuda M, Hiraki Y (1998) Sequential progression of the differentiation program by bone morphogenetic protein-2 in chondrogenic cell line ATDC5. Exp Cell Res 241:1–11
Ede DA (1983) Cellular condensations and chondrogenesis. In: Hall BK (ed) Cartilage, vol 2. Academic Press, New York, pp 143–185
Lefebvre V, Huang W, Harly VR, Goodfellow PN, de crombrugghe B (1997) Sox-9 is a potent activator of the chondrocyte-specific enhancer of the proa1(II) collagen gene. Mol Cell Biol 17:2336–2346
Ng LJ, Wheatly S, Muscat GE, Conway-Campbell J, Bowles J, Wright E et al (1997) Sox9 binds DNA, activates transcription, and coexpression with type II collagen during chondrogenesis in the mouse. Dev Biol 183:108–121
Ducy P, Zang R, Geoffroy V, Ridall AL, Karsenty G (1997) Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89:747–754
Inada M, Yasui T, Nomura S, Deguchi K, Himeno M et al (1999) Maturational disturbance of chondrocytes in cbfa1-deficient mice. Dev Dyn 214:279–290
Hall BK, Miyake T (2000) All for one and one for all: condensations and the initiation of skeletal development. Bioessays 22:138–147
Nah HD, Rodgers BJ, Kulyk WM, Kream BE, Kosher RA, Upholt WB (1988) In situ hybridization analysis of the expression of the type II collagen gene in the developing chicken limb bud. Coll Relat Res 8:277–294
DeLise AM, Fischer L, Tuan RS (2000) Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage 8:309–334
Oberlender SA, Tuan RS (1994) Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development 120:177–187
Haas AR, Tuan RS (1999) Chondrogenic differentiation of murine C3H10T1/2 multipotential mesenchymal cells: II. Stimulation by bone morphogenetic protein-2 requires modulation of N-cadherin expression and function. Differentiation 64:77–89
Tufan AC, Tuan RS (2001) Wnt regulation of limb mesenchymal chondrogenesis is accompanied by altered N-cadherin-related functions. FASEB J 15:1436–1438
Kawai J, Akiyama H, Shigeno C, Ito H, Konishi J, Nakamura T (1999) Effects of transforming growth factor-beta signaling on chondrogenesis in mouse chondrogenic EC cells, ATDC5. Eur J Cell Biol 78:707–714
Acknowledgments
This work was supported in part by the Grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. The monoclonal antibody developed by Bruce Carterson was obtained from Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Anraku, Y., Mizuta, H., Sei, A. et al. Analyses of early events during chondrogenic repair in rat full-thickness articular cartilage defects. J Bone Miner Metab 27, 272–286 (2009). https://doi.org/10.1007/s00774-009-0038-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-009-0038-x