Abstract
Introduction
Idiopathic scoliosis, defined as a > 10° curvature of the spine in the frontal plane, is one of the most common spinal deformities. Age, initial curve magnitude and other parameters define whether a scoliotic deformity will progress or not. Still, their interactions and amounts of individual contribution are not fully elaborated and were the aim of this systematic review.
Methods
A systematic literature search was conducted in the common databases using MESH terms, searching for predictive factors of curve progression in adolescent idiopathic scoliosis (“adolescent idiopathic scoliosis” OR “ais” OR “idiopathic scoliosis”) AND (“predictive factors” OR “progression” OR “curve progression” OR “prediction” OR “prognosis”). The identified and analysed factors of each study were rated to design a top five scale of the most relevant factors.
Results
Twenty-eight investigations with 8255 patients were identified by literature search. Patient-specific risk factors for curve progression from initial curve were age (at diagnosis < 13 years), family history, bone mineral status (< 110 mg/cm3 in quantitative CT) and height velocity (7–8 cm/year, peak 11.6 ± 1.4 years). Relevant radiological criteria indicating curve progression included skeletal maturity, marked by Risser stages (Risser < 1) or Sanders Maturity Scale (SMS < 5), the initial extent of the Cobb angle (> 25° progression) and curve location (thoracic single or double curve).
Discussion
This systematic review summarised the current state of knowledge as the basis for creation of patient-specific algorithms regarding a risk calculation for a progressive scoliotic deformity. Curve magnitude is the most relevant predictive factor, followed by status of skeletal maturity and curve location.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic scoliosis, defined as a > 10° curvature of the spine in the frontal plane, is one of the most common spinal deformities. Ninety per cent of all idiopathic scoliosis occurs during adolescence with a prevalence of 1–2% [1, 2]. Most patients are female, at a ratio of 1.5:1 (female/male) in mild scoliosis and a ratio of up to 10:1 (f:m) in scoliosis, with a Cobb angle > 30° [1].
Risk factors for curve progression have been widely analysed with partially inconsistent findings [3,4,5]. Specifically, personal characteristics, such as sex, age, menarche status and bone mineral density (BMD), are still debated controversially. In a majority of studies, risk factors, such as initial magnitude of curve at first presentation and skeletal maturity status and curve presentation at peak height velocity (PHV), seem to show the highest correlation to curve progression [6, 7]. Nevertheless, most studies are limited due to certain factors, i.e., inclusion of only female patients, only skeletal immature patients, or treated patients without a control group. Moreover, many studies differ in the definition of progress, either as an increase in the analysed Cobb angle or until surgical treatment is obtained. In addition, the categorisation of patients along skeletal maturity scales (SMS, Risser sign), as well as determination of PHV are challenging.
Beyond the above-mentioned issues, the interactions of risk factors and amount of individual contribution to curve progression have not yet been fully elaborated. While age is obviously interacting with nearly every other risk factor, other factors seem to be independent, such as BMD or gender. In a previously published systematic review, Noshenkho et al. [8] identified 25 investigations yielding eight clinically assessable risk factors of curve severity or progression of adolescent idiopathic scoliosis (AIS). Furthermore, they reported nearly one-third of patients with brace treatment progressed and approximately 15% finally required surgical correction. However, they only found low evidence, due to limitations of the included studies and since no method for prediction of clinical use could be elaborated [8].
Therefore, the aim of this systematic review was to identify the most contributing patient-specific and radiological risk factors and their influence on curve progression. On the basis of the results, further investigations should be carried out with the overall aim to develop an artificial intelligence (AI) algorithm to predict progression of spinal curve at the time of initial clinical presentation.
Materials and methods
Study design
We conducted a comprehensive systematic review of the literature, according to PRISMA guidelines, including the PubMed and Google scholar databases [9]. The keywords used in the database search were (“adolescent idiopathic scoliosis” OR “ais” OR “idiopathic scoliosis”) AND (“predictive factors” OR “progression” OR “curve progression” OR “prediction” OR “prognosis”), (english OR german).
Inclusion and study selection
All studies included were either in the English or German language. The period of analysis was set from 2010 to 2020 to gain new aspects in influencing factors. The authors limited the research to observational and interventional studies. Titles and abstracts were reviewed by two authors (ML and MJS), independently. Duplicates were removed and full texts were screened for suitability. Systematic reviews, meta-analyses as well as reviews, case reports and expert opinion articles were excluded from analysis. Moreover, studies analysing genetic or cellular risk factors were excluded.
Data analysis
Data regarding study design, year of publication, author names, number of patients, sex, age, hereditary, skeletal maturity, initial curve magnitude (as measured by the method of Cobb [10]), type of curve, growth velocity (body height velocity and spinal growth velocity) and progression of curve were extracted. Data were subdivided into (i) patient-specific factors and (ii) radiological parameters for interpretation. The level of evidence was defined based on the suggestions of evidence levels for orthopaedic journals by Slobogean et al. [11]. Where a level of evidence was already provided, it was either adopted or adjusted, if necessary.
Statistical analysis
After thorough discussion with a statistician following statistical measurements, further meta-analysis of the data was rejected due to the large heterogeneity of the studies and limited data provided in some investigations. We identified the top predictive factors of the included studies (if the study examined only one factor, it was regarded as the top factor) and generated a list of the five most relevant predictive factors in AIS.
Results
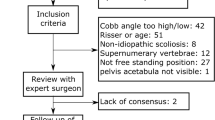
The initial research identified 1772 potentially eligible publications in both databases. After screening for titles and abstracts, 76 studies were left for analysis. After full text analysis, 28 studies were finally included in the systematic review. The further selection process is outlined by PRISMA flow chart in Fig. 1. In summary, 28 studies with 8.255 patients were identified, including patient-specific and radiological parameters. Twenty-one were retrospective investigations, five prospective and two biomedical simulation studies. Table 1 shows the studies included for analysis and their characteristics. The mean level of evidence of all studies was 3.08.
By analysing the studies’ most examined factors and their individual contribution sorted within the studies, we generated a list of the top five factors that influence and predict curve progression in AIS. This Top 5-list of identified risk factors influencing curve progression included:
-
(1)
Curve magnitude (initial presentation),
-
(2)
Skeletal maturity (Risser, SMS, proximal humerus, distal radius/ulna),
-
(3)
Curve location (thoracic or double-thoracic),
-
(4)
Age,
-
(5)
Status of menarche.
Patient-specific risk factors
Age
Age is one of the most important factors, as it is naturally linked to growth, skeletal maturity and consecutively to the onset of menarche in female patients. In their retrospective cohort study including 1464 patients, Lee et al. [6] stated that age was a relevant prognostic factor, with the age at risk of < 11.3 years. The impact of age decreased with age and height. Using Cox regression models, moderate effects between age and gender and age and initial curve were found.
PHV
Chazono et al. [13] published data of 56 skeletal immature patients reporting median PHV as 8.5 cm/year (non-surgical group [NS]) and 8.9 cm/year (surgical group [S]). A height velocity of > 7 cm/year suggested onset of PHV. Chronological age at PHV (APHV) was 11.9 and 11 years (NS vs. S group, respectively), narrowing PHV down to ages 11–12 years. Height at PHV (HPVH) was 152.9 and 149.3 (NS vs. S group, respectively).
Family history
With numerous findings regarding hereditary aspects in AIS, the cause of scoliosis seems to rely on several genetic aspects. Grauers et al. [18] published data of 1463 patients revealing a significant difference in maximum curve size between patients with one or more relative (any kind) with scoliosis (median = 35°, interquartile range = 25) and patients without any relative with scoliosis (median = 32°, interquartile range = 23) (p = 0.022). Moreover, the odds ratio (OR; 95% confidence interval [CI]) for having a Cobb angle of > 40° or more was 1.30 (1.05–1.6) if the patient had a family history of scoliosis [18].
Gender
Gender as a risk factor continues to be debated, in regards to curve progression. Smorgick et al. [33] presented data of 163 patients with indications for surgical correction and could not find significant differences in curve severity or progression of curve.
BMD
Regarding BMD, two studies were found for analysis. Yip et al. [36] published data of 513 patients, with 169 subjects displaying osteopenia with a mean follow-up of 4.6 years. Osteopenic AIS patients had a significantly later menarche age (12.2 vs. 13.1 years) and taller standing heights (155.7 cm vs. 152.7 cm). Following univariate and multivariate analysis, the risk of progression decreased with maturity (age and menarche status), but increased with initial curve magnitude. With significant difference, osteopenic AIS patients have a twofold higher risk of progressing to surgical level (hazard ratio [HR] = 2.25, 95% CI = 1.2–4.2). In 90 patients receiving high-resolution peripheral quantitative computed tomography (HR-pQCT) of distal radius, cut-off values of Cobb angle > 24° and Dcort (volumetric density of cortical bone measured at distal radius) less than 570 mg HA/cm3 were found to showing curve progression. A biomedical simulation study supporting the role of BMD in AIS was published by Song et al. [38]. Their finite element model, based on a 14-year-old male patient with Lenke Type 1 curve and Cobb angle of 31°, showed curve progression when grading the bone mineral status in normal, osteopenia and middle-grade osteoporosis (200, 110 and 50 mg/cm3 in quantitative CT, respectively).
Hump dimensions
Aulisa et al. [12] found a significant correlation of hump dimensions and curve severity at the beginning and end of brace treatment in an investigation of 134 patients, except of lumbar curves at baseline. The change of hump dimensions was more evident than the change of curve correction.
Radiological parameters
Initial curve magnitude, curve location and form of curve
In a risk classification study, Lee et al. [6] found initial curve magnitude to be the most important factor for risk of progression in a set of 1464 subjects. At highest risk were patients with Cobb angle > 26° (HR = 8.8), while lowest risk patients were recorded with an initial Cobb angle < 18° (HR = 1) [6]. Initial curve magnitude was seen as a relevant predictive factor by Sitoula et al. [32]. They found that in Sanders Stage 2 (SS), patients with an initial Cobb angle > 25° progressed, and SS1 and SS3 patients with initial Cobb angles > 35° also progressed [32]. All patients with initial Cobb angles > 40° progressed, however, none of the patients with initial Cobb angles < 30° in SS 5–7 showed progression [32]. Lara et al. [21] published retrospective data of 72 patients of African-American background who were assessed for curve progression in AIS. Higher curve magnitude at presentation was significantly associated with further curve progression [21]. This finding was also supported by Cheung et al. [15]. Performing receiver operating characteristics (ROC) analysis for PHV and curve magnitude and form, Chazono et al. [13] report an initial Cobb angle of 31.5° at PHV as the cut-off for progression in single curve and 30° in double curves. Zapata et al. [37] found similar progression rates (> 5° Cobb angle) in skeletal immature patients (Risser 0 and 1) and mild forms of scoliosis. Patients with curves 20–24° did not progress significantly more than patients with curves 15–19° (mean progression of 10° vs. 9°).
Dolan et al. [16] found a higher risk of progression when one or more thoracic curves was found at initial presentation. Morrison et al. [25] demonstrated a significant correlation of spinous process angle (SPA) with Cobb angle, as well as apical vertebrae rotation (AVR). Therefore, SPA and AVR represent further radiological parameter that predict Cobb angle. Ohashi et al. [29] found significant curve progression in patients with higher L3 and L4 tilt (> 16°) in skeletal maturity. Guo et al. [20] published data regarding 60 patients with bracing showing that mean pelvic tilt, T1-spinopelvic inclination and T9-spinopelvic inclination angles were independent predictors for curve progression. Pre-bracing pelvic tilt ≤ −0.5° was strongly predictive and T1-spinopelvic inclination ≤ 3.5° was moderately predictive in curve progression during brace treatment. Regarding curve flexibility in bending x-rays, Chen et al. [14] demonstrated that in patients with Risser < 5, Cobb angle and curve location were significantly related to curve flexibility, whereas in Risser = 5, the reduction in flexibility increased. Drevelle et al. [17] presented a biomedical simulation study evaluation 12 patients with mild scoliosis and six asymptomatic ones. After 3D reconstruction of the spine, a patient-specific finite element model was used. They found that in pre-existing scoliosis, anterior spinal growth with a decrease of disc mechanical stiffness could lead to progression of scoliosis [17]. Further spinal parameters were assessed by Nault et al. [27], who analysed the spinal morphology data of 37 patients using 2D and 3D computerised measurements. They stated that disc wedging is followed by the vertebral body.
Annual curve progression (ACP)
Several studies analysed an ACP defining the growth of the Cobb angle over one year. In a prospective cohort study, Ward et al. [35] found ACP was not linear over time in curves > 40° Cobb angles in 738 patients. Mean ACP was 6.3 ± 10.4°, yet, in the first year of follow-up, ACP was 11.5. After 1–2 years of follow-up, ACP was 8.2 ± 8.8° and at 2–5 years, ACP was 3.7 ± 4.1°, with average follow-up of 3.2 years. The ACP is supposed to be at its maximum immediately after the Cobb angle reaches 40°. In the literature, a progression rate of > 1° of the Cobb angle in cases of severe scoliosis (Cobb > 40°) is widely accepted [4, 39, 40]. Ohashi et al. [29] presented a general ACP of 0.41°/year in cases of > 40° Cobb angle and skeletal maturity with a follow-up of 25 years (± 6.9 years). Grothaus et al. [19] published an ACP of 2.3°/year in a follow-up of 2 years in patients with > SS7 and < 50° at initial examination. Ramo et al. [30] presented the data of skeletal immature patients with a delay for surgical correction of severe scoliosis, which, in 6 months’ progression in Risser 0 was 1.6°/month, while in Risser 1–5, it was only 0.4°/month.
Skeletal maturity
In a validation study, Sitoula et al. [32] found a strong predictive correlation between SS and initial Cobb angle in prediction of curve progression in AIS. In 161 patients, curve progression > 50° was found in 58 patients. Curve progression in SS2 patients with initial Cobb angles > 25° progressed, while in SS1 and SS3 patients with initial Cobb angles > 35° progressed. All patients, regardless of SS with initial Cobb angles > 40°, progressed. In their prospective study, Cheung et al. [15] found Risser 0 as significant predictor of surgical threshold, but the classification up to Risser 3 are still at risk for progression, despite inability to set up a significant prediction model for Risser stages. In 2010, Nault et al. [26] published matching European and US Risser stages in 100 female patients. Although moderate agreement between the US and European grading systems was seen, Risser stages were not good predictors of the curve acceleration phase. They introduced a new group, Risser 0 with closed triradiate cartilage, and Risser 1, which were the best predictors of the beginning of the acceleration phase. Triradiate cartilage was considered to be another sign classifying skeletal maturity, however, triradiate cartilage cannot be graded in to steps.
Cheung et al. [15] analysed prediction of curve progression using the distal radius and ulna (DRU) classification, displaying that at stage R6/U5, curves are likely to progress > 40° in Cobb angle when initial Cobb angle was approximately 25°. In initial curves of ≥ 35°, regardless of skeletal maturity, progression was found. In the case–control study of Dolan et al. [16] with 115 patients validating the SMS scale for skeletal maturity, it was demonstrated that untreated patients were much more likely to progress to severe scoliosis. At high risk of progression were patients with an SMS of 1–2 and Cobb angles of > 16°. Differentiation in SMS2 and SMS3 patients, however, seems to be essential, due to timing of PHV.
In addition to Risser’s sign and SMS, the status of the triradiate cartilage is another marker of skeletal maturity. Ramo et al. [30] marked open triradiate cartilage as a significant risk factor for severe curve progression. Wang et al. [34] presented the radius dimension ratio (Radius Diameter/Radius Lenght), showing significantly lower ratios in patients with severe AIS and a ratio that correlated with curve severity (r = −0.120; p = 0.039). In 2019, Li et al. [23] published data of 216 patients, presenting a new classification in skeletal maturity according to the stages of humeral head ossification. They found significant reliability in intra-observer and inter-observer correlation coefficients of 0.97 and 0.92, respectively, among eight investigators. The combination of the proximal humeral ossification system and Sanders Hand System was capable of predicting PHV more accurately, compared to a combination of Risser and triradiate closure. In 2020, Li et al. [24] presented the DRU scoring system analysing ossification. They demonstrated a significant correlation combining radius and ulna scores in 40 patients, predicting PHV with an intra-observer score of 0.94 and inter-observer reliability of 0.93.
Discussion
Idiopathic scoliosis is the most common spinal deformity seen in adolescence with severe sequelae, and if curve progression is not stopped, the need for surgical correction is often inevitable. At a patient’s initial presentation, prediction of further curve progression is key in managing therapy. An algorithm for risk analysis of curve progression would serve as a great support for patients and their families. In our systematic review, we found several predictive factors that were correlated with a higher risk of curve progression. In particular, initial curve magnitude and status of skeletal maturity were the most relevant factors to predict curve progression, followed by curve location, age and status of menarche. However, these factors are still considered independently and their interactions have not yet been further investigated. When developing an algorithm for risk analysis in curve progression of AIS, the focus should be on radiological parameter accessible during clinic visits requiring the least radiation as possible.
Focusing on radiological parameters, the initial curve magnitude at presentation seems to be one of the most important risk factors for curve progression (Table 2). With several studies highlighting the significant correlation of initial curve magnitude with risk for progression, initial curve magnitude is a radiological parameter that is easy to diagnose in standard radiographs. In the studies dealing with initial curve magnitude that were included in this analysis, a Cobb angle > 25–28° at initial presentation was mainly correlated to curve progression to > 50° of Cobb or to surgical intervention. Certainly, as the patients in adolescence are still in the growth phase, the actual curve progression also depends on skeletal maturity.
Interestingly, the curve location, which is also easily diagnosed, was found significant in the investigation of Dolan et al. [16]. In their Akaike information criterion (AIC) model, they presented that thoracic curves were more likely to progress than lumbar or thoracolumbar curves (OR = 4.09). However, two other studies did not find any significance in curve location [32, 21].
Skeletal maturity is another major risk factor in terms of progression [13, 32, 15, 16, 23, 24]. Controversial findings exist regarding the actual categorisation mechanism, with numerous studies highlighting inadequate predictability of further growth by Risser stages, and Risser classification being dependent on descent or bone mineral status [26]. Tanner and Whitehouse used DRU scores to establish a different method for determining skeletal maturity in adolescence [41]. Sanders, however, reported the lowest correlation of radius/ulna scores to curve acceleration phase [42], and DRU scores were modified by Luk et al. [43]. Neal et al. [28] documented a higher correlation to skeletal ossification based on SMS rather than Risser, but Risser is still significant, as confirmed by Sitoula et al. [32], who reported significant predictive probability for SMS and Risser stages. Moreover, recently published additional classification systems, such as the distal radius and ulna score, as well as the proximal humerus ossification scale, may allow for even further evaluation of stages at risk, as demonstrated by Li et al. [23, 24].
With regard to additional radiological parameters, specifically sagittal parameters, such as L3/4 tilt, pelvic tilt, T1 and T9 spinopelvic inclination, SPA and apical vertebrae angle, are parameters easily to analyse in standard radiographs and do not require additional radiation. These new parameters should be evaluated further in prospective studies.
Patient-specific risk factors
Identified patient-specific risk factors included age (age at diagnosis < 13 years), positive family history, bone quality and mineral status. The trend regarding female patients exhibiting earlier onset of scoliosis and higher prevalence of greater curve magnitude may be in contrast to the actual risk of curve progression, which seems not to be gender dependent. Smorgick et al. [33] could not demonstrate a significant difference in gender regarding curve severity or progression. The authors did, however, postulate a male trend of lower thoracic flexibility, compared to female patients, assumingly increasing the failure rate of brace treatment and less effective surgical correction [44]. These findings are consistent with previous studies on gender regarding scoliosis severity [45, 46]. Therefore, gender cannot be seen as significant risk factor for severe curve progression. Regarding bone quality, based on the clinical data of Yip et al. [36] and the simulation data of Song et al. [38], BMD seems to be a consistent risk factor for curve progression in AIS. However, both studies have limitations, as activity levels and nutrition levels were not analysed. As a result, the concern of BMD causing AIS or scoliosis and back pain, leading to decreased activity resulting in lower BMD, remains elusive. In patients with positive family history, more severe curves are seen in patients with one family member. With regard to the top five predictive factors, age is the one factor that influences status of skeletal maturity and menarche. Patient-specific risk factors, however, seem to be more of a yes–no scheme in terms of risk, although radiological factors present a more significant risk.
Limitations
This systematic review should identify all radiological parameters associated to the risk of curve progression and may be used as clinical markers on standardised radiographs. The mean level of evidence of the included studies is 3.08. As recently published by Noshenkho et al., the study’s characteristics show high heterogeneity and level of evidence remains low in observational studies. The paucity of good, randomised controlled clinical studies reveals the necessity to increase research in this field and additional studies should mainly include the predictive factors found by our systematic review.
Conclusion
In summary, several risk factors of curve progression in AIS have been identified in recent years. Especially, radiological parameters, which are more objective, seem to have highest value in predicting risk of curve progression. Summarising the majority of parameters showing significance, the most relevant factors found are the initial curve magnitude and status of skeletal maturity. These two factors may successfully predict the risk of progression of AIS. Factors such as age, status of menarche, BMD and other radiographic parameters might influence the amount of curve progression and should be reflected as well. Unfortunately, no further meta-analysis was possible due to high heterogeneity and lack of evidence within the included studies. Hence, new investigations regarding this topic should analyse the main factors identified thus far. Finally, designing an AI model based on patient-specific factors and radiological parameters calculating individual risk of progression would be desirable.
References
Konieczny MR, Senyurt H, Krauspe R (2013) Epidemiology of adolescent idiopathic scoliosis. J Child Orthop 7(1):3–9. https://doi.org/10.1007/s11832-012-0457-4
Reamy BV, Slakey JB (2001) Adolescent idiopathic scoliosis: review and current concepts. Am Fam Physician 64(1):111–116
Tan KJ, Moe MM, Vaithinathan R, Wong HK (2009) Curve progression in idiopathic scoliosis: follow-up study to skeletal maturity. Spine 34(7):697–700. https://doi.org/10.1097/BRS.0b013e31819c9431
Weinstein SL, Ponseti IV (1983) Curve progression in idiopathic scoliosis. J Bone Joint Surg Am 65(4):447–455
Charles YP, Daures JP, de Rosa V, Dimeglio A (2006) Progression risk of idiopathic juvenile scoliosis during pubertal growth. Spine 31(17):1933–1942. https://doi.org/10.1097/01.brs.0000229230.68870.97
Lee CF, Fong DY, Cheung KM, Cheng JC, Ng BK, Lam TP, Yip PS, Luk KD (2012) A new risk classification rule for curve progression in adolescent idiopathic scoliosis. Spine J Off J North Am Spine Soc 12(11):989–995. https://doi.org/10.1016/j.spinee.2012.05.009
Charles YP, Canavese F, Dimeglio A (2017) Curve progression risk in a mixed series of braced and nonbraced patients with idiopathic scoliosis related to skeletal maturity assessment on the olecranon. J Pediatr Orthop B 26(3):240–244. https://doi.org/10.1097/BPB.0000000000000410
Noshchenko A, Hoffecker L, Lindley EM, Burger EL, Cain CM, Patel VV, Bradford AP (2015) Predictors of spine deformity progression in adolescent idiopathic scoliosis: a systematic review with meta-analysis. World J Orthop 6(7):537–558. https://doi.org/10.5312/wjo.v6.i7.537
Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8(5):336–341. https://doi.org/10.1016/j.ijsu.2010.02.007
Cobb J (1948) Outline for the study of scoliosis. Instr Course Lect AAOS 5:261–275
Slobogean G, Bhandari M (2012) Introducing levels of evidence to the Journal of Orthopaedic Trauma: implementation and future directions. J Orthop Trauma 26(3):127–128. https://doi.org/10.1097/BOT.0b013e318247c931
Aulisa AG, Guzzanti V, Perisano C, Marzetti E, Menghi A, Giordano M, Aulisa L (2018) Correlation between hump dimensions and curve severity in idiopathic scoliosis before and after conservative treatment. Spine 43(2):114–119. https://doi.org/10.1097/BRS.0b013e3181ee77f9
Chazono M, Tanaka T, Marumo K, Kono K, Suzuki N (2015) Significance of peak height velocity as a predictive factor for curve progression in patients with idiopathic scoliosis. Scoliosis 10(Suppl 2):S5. https://doi.org/10.1186/1748-7161-10-S2-S5
Chen ZQ, Zhao YF, Bai YS, Liu KP, He SS, Wang CF, Zhang JT, Li M (2011) Factors affecting curve flexibility in skeletally immature and mature idiopathic scoliosis. J Orthop Sci 16(2):133–138. https://doi.org/10.1007/s00776-011-0034-6
Cheung JPY, Cheung PWH, Samartzis D, Luk KD (2018) APSS-ASJ best clinical research award: predictability of curve progression in adolescent idiopathic scoliosis using the distal radius and ulna classification. Asian Spine J 12(2):202–213. https://doi.org/10.4184/asj.2018.12.2.202
Dolan LA, Weinstein SL, Abel MF, Bosch PP, Dobbs MB, Farber TO, Halsey MF, Hresko MT, Krengel WF, Mehlman CT, Sanders JO, Schwend RM, Shah SA, Verma K (2019) Bracing in Adolescent Idiopathic Scoliosis Trial (BrAIST): Development and Validation of a Prognostic Model in Untreated Adolescent Idiopathic Scoliosis Using the Simplified Skeletal Maturity System. Spine Deform 7(6):890-898.e4. https://doi.org/10.1016/j.jspd.2019.01.011
Drevelle X, Lafon Y, Ebermeyer E, Courtois I, Dubousset J, Skalli W (2010) Analysis of idiopathic scoliosis progression by using numerical simulation. Spine 35(10):E407-412. https://doi.org/10.1097/BRS.0b013e3181cb46d6
Grauers A, Danielsson A, Karlsson M, Ohlin A, Gerdhem P (2013) Family history and its association to curve size and treatment in 1463 patients with idiopathic scoliosis. Eur Spine J Off PublEur Spine SocEur Spinal Deform SocEur Sect Cerv Spine Res Soc 22(11):2421–2426. https://doi.org/10.1007/s00586-013-2860-z
Grothaus O, Molina D, Jacobs C, Talwalkar V, Iwinski H, Muchow R (2020) Is it growth or natural history? increasing spinal deformity after sanders stage 7 in females with AIS. J PediatrOrthop 40(3):e176–e181. https://doi.org/10.1097/BPO.0000000000001415
Guo J, Liu Z, Lv F, Zhu Z, Qian B, Zhang X, Lin X, Sun X, Qiu Y (2012) Pelvic tilt and trunk inclination: new predictive factors in curve progression during the Milwaukee bracing for adolescent idiopathic scoliosis. Eur Spine J Off PublEur Spine SocEur Spinal Deform SocEur Sect Cerv Spine Res Soc 21(10):2050–2058. https://doi.org/10.1007/s00586-012-2409-6
Lara T, Astur N, Jones TL, Perake V, Moisan A, Warner WC Jr, Sawyer JR, Kelly DM (2017) The risk of curve progression and surgery in African Americans with adolescent idiopathic Scoliosis. Spine Deform 5(4):250–254. https://doi.org/10.1016/j.jspd.2017.01.013
Li Z, Li G, Chen C, Li Y, Yang C, Li M, Xu W, Zhu X (2017) The radiographic parameter risk factors of rapid curve progression in Lenke 5 and 6 adolescent idiopathic scoliosis: a retrospective study. Medicine (Baltimore) 96(52):e9425. https://doi.org/10.1097/MD.0000000000009425
Li DT, Linderman GC, Cui JJ, DeVries S, Nicholson AD, Li E, Petit L, Kahan JB, Talty R, Kluger Y, Cooperman DR, Smith BG (2019) The proximal humeral ossification system improves assessment of maturity in patients with Scoliosis. J Bone Joint Surg Am 101(20):1868–1874. https://doi.org/10.2106/JBJS.19.00296
Li Y, Mao S, Shi B, Liu Z, Liu D, Sun X, Qiu Y, Zhu Z (2020) Utilization of distal radius and ulna classification scheme in predicting growth peak and curve progression in idiopathic scoliosis girls undergoing bracing treatment. Eur Spine J Off PublEur Spine SocEur Spinal Deform SocEur Sect Cerv Spine Res Soc 29(4):770–778. https://doi.org/10.1007/s00586-020-06289-8
Morrison DG, Chan A, Hill D, Parent EC, Lou EH (2015) Correlation between Cobb angle, spinous process angle (SPA) and apical vertebrae rotation (AVR) on posteroanterior radiographs in adolescent idiopathic scoliosis (AIS). Eur Spine J Off PublEur Spine SocEur Spinal Deform SocEur Sect Cerv Spine Res Soc 24(2):306–312. https://doi.org/10.1007/s00586-014-3684-1
Nault ML, Parent S, Phan P, Roy-Beaudry M, Labelle H, Rivard M (2010) A modified Risser grading system predicts the curve acceleration phase of female adolescent idiopathic scoliosis. J Bone Joint Surg Am 92(5):1073–1081. https://doi.org/10.2106/JBJS.H.01759
Nault ML, Mac-Thiong JM, Roy-Beaudry M, deGuise J, Labelle H, Parent S (2013) Three-dimensional spine parameters can differentiate between progressive and nonprogressive patients with AIS at the initial visit: a retrospective analysis. J Pediatr Orthop 33(6):618–623. https://doi.org/10.1097/BPO.0b013e318292462a
Neal KM, Shirley ED, Kiebzak GM (2018) Maturity indicators and adolescent idiopathic Scoliosis: evaluation of the sanders maturity scale. Spine 43(7):E406–E412. https://doi.org/10.1097/BRS.0000000000002483
Ohashi M, Watanabe K, Hirano T, Hasegawa K, Katsumi K, Shoji H, Mizouchi T, Endo N (2018) Predicting factors at skeletal maturity for curve progression and low back pain in adult patients treated nonoperatively for adolescent idiopathic scoliosis with thoracolumbar/lumbar curves: a mean 25-year follow-up. Spine 43(23):E1403–E1411. https://doi.org/10.1097/BRS.0000000000002716
Ramo B, Tran DP, Reddy A, Brown K, Niswander C, Erickson M, Garg S (2019) Delay to surgery greater than 6 months leads to substantial deformity progression and increased intervention in immature adolescent idiopathic scoliosis (AIS) patients: a retrospective cohort study. Spine Deform 7(3):428–435. https://doi.org/10.1016/j.jspd.2018.09.012
Shi B, Mao S, Liu Z, Sun X, Zhu Z, Zhu F, Cheng JC, Qiu Y (2016) Spinal growth velocity versus height velocity in predicting curve progression in peri-pubertal girls with idiopathic scoliosis. BMC Musculoskelet Disord 17(1):368. https://doi.org/10.1186/s12891-016-1221-6
Sitoula P, Verma K, Holmes L Jr, Gabos PG, Sanders JO, Yorgova P, Neiss G, Rogers K, Shah SA (2015) Prediction of curve progression in idiopathic scoliosis: validation of the sanders skeletal maturity staging system. Spine 40(13):1006–1013. https://doi.org/10.1097/BRS.0000000000000952
Smorgick Y, Nassar M, Tamir E, Tal S, Mirovsky Y, Anekstein Y (2019) Clinical and radiographical characteristics in male and female adolescent idiopathic scoliosis surgical candidates. Isr Med Assoc J 21(3):213–216
Wang WJ, Hung VW, Lam TP, Ng BK, Qin L, Lee KM, Qiu Y, Cheng JC, Yeung HY (2010) The association of disproportionate skeletal growth and abnormal radius dimension ratio with curve severity in adolescent idiopathic scoliosis. Eur Spine J Off PublEur Spine SocEur Spinal Deform SocEur Sect Cerv Spine Res Soc 19(5):726–731. https://doi.org/10.1007/s00586-009-1247-7
Ward WT, Crasto JA, Kenkre TS, Dede O, Bosch PP, Roach JW (2020) Intermediate-term annualized curve progression of adolescent idiopathic scoliosis curves measuring 40 degrees or greater. Spine Deform 8(4):629–636. https://doi.org/10.1007/s43390-020-00088-3
Yip BHK, Yu FWP, Wang Z, Hung VWY, Lam TP, Ng BKW, Zhu F, Cheng JCY (2016) Prognostic value of bone mineral density on curve progression: a longitudinal cohort study of 513 girls with adolescent idiopathic scoliosis. Sci Rep 6:39220. https://doi.org/10.1038/srep39220
Zapata KA, Sucato DJ, Lee MC, Jo CH (2019) Skeletally immature patients with adolescent idiopathic scoliosis curves 15 degrees -24 degrees are at high risk for progression. Spine Deform 7(6):870–874. https://doi.org/10.1016/j.jspd.2019.02.012
Song XX, Jin LY, Li XF, Qian L, Shen HX, Liu ZD, Yu BW (2018) Effects of low bone mineral status on biomechanical characteristics in idiopathic scoliotic spinal deformity. World Neurosurg 110:e321–e329. https://doi.org/10.1016/j.wneu.2017.10.177
Agabegi SS, Kazemi N, Sturm PF, Mehlman CT (2015) Natural history of adolescent idiopathic scoliosis in skeletally mature patients: a critical review. J Am AcadOrthopSurg 23(12):714–723. https://doi.org/10.5435/JAAOS-D-14-00037
Weinstein SL, Dolan LA (2015) The evidence base for the prognosis and treatment of adolescent idiopathic scoliosis: the 2015 orthopaedic research and education foundation clinical research award. J Bone Joint Surg Am 97(22):1899–1903. https://doi.org/10.2106/JBJS.O.00330
Tanner JM, Whitehouse RH (1976) Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child 51(3):170–179. https://doi.org/10.1136/adc.51.3.170
Sanders JO, Browne RH, McConnell SJ, Margraf SA, Cooney TE, Finegold DN (2007) Maturity assessment and curve progression in girls with idiopathic scoliosis. J Bone Joint Surg Am 89(1):64–73. https://doi.org/10.2106/JBJS.F.00067
Luk KD, Saw LB, Grozman S, Cheung KM, Samartzis D (2014) Assessment of skeletal maturity in scoliosis patients to determine clinical management: a new classification scheme using distal radius and ulna radiographs. Spine J Off J North Am Spine Soc 14(2):315–325. https://doi.org/10.1016/j.spinee.2013.10.045
Marks M, Petcharaporn M, Betz RR, Clements D, Lenke L, Newton PO (2007) Outcomes of surgical treatment in male versus female adolescent idiopathic scoliosis patients. Spine 32(5):544–549. https://doi.org/10.1097/01.brs.0000256908.51822.6e
Sponseller PD, Flynn JM, Newton PO, Marks MC, Bastrom TP, Petcharaporn M, McElroy MJ, Lonner BS, Betz RR, Harms Study Group (2012) The association of patient characteristics and spinal curve parameters with Lenke classification types. Spine 37(13):1138–1141. https://doi.org/10.1097/BRS.0b013e31824054a5
Wang W, Zhu Z, Zhu F, Sun C, Wang Z, Sun X, Qiu Y (2012) Different curve pattern and other radiographical characteristics in male and female patients with adolescent idiopathic scoliosis. Spine 37(18):1586–1592. https://doi.org/10.1097/BRS.0b013e3182511d0c
Funding
Open Access funding enabled and organized by Projekt DEAL. No funding applicable.
Author information
Authors and Affiliations
Contributions
MJS, JB and PE had the idea for the article, ML, AH and PF performed the literature search and data analysis. ML and MJS drafted the manuscript. SO, MF and PE critically revised the work.
Corresponding author
Ethics declarations
Conflict of interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lenz, M., Oikonomidis, S., Harland, A. et al. Scoliosis and Prognosis—a systematic review regarding patient-specific and radiological predictive factors for curve progression. Eur Spine J 30, 1813–1822 (2021). https://doi.org/10.1007/s00586-021-06817-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-021-06817-0