Abstract
Purpose
To investigate the prevalence of and to identify independent predictors associated with scoliosis in patients with multiple hereditary exostoses (MHE).
Methods
Fifty patients with MHE were clinically examined, and the diagnosis of scoliosis was made based on radiographs. To classify disease severity, three classes based on the presence of deformities and functional limitations were defined. Significant independent predictors of scoliosis in MHE were statistically analyzed.
Results
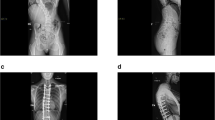
Scoliosis was present in 36 patients (MHE-scoliosis) (72 %). In the MHE-scoliosis group, the mean primary curve was 15.3° ± 5.7° (range 10°–34°) and the mean minor curve was 10.6° ± 7° (range 6°–32°). Left curve was predominant (72 %), and the apex was located in the thoracolumbar or lumbar spine in 64 % of patients. Univariable and multivariable analyses confirmed that MHE severity was a significant predictor of moderate scoliosis (≥20°).
Conclusions
Our study confirmed that scoliosis is a common feature of MHE and disease severity is a predictor of moderate scoliosis (≥20°).



Similar content being viewed by others
References
Matsumoto K, Irie F, Mackem S, Yamaguchi Y (2010) A mouse model of chondrocyte-specific somatic mutation reveals a role for Ext1 loss of heterozygosity in multiple hereditary exostoses. Proc Natl Acad Sci USA 107:10932–10937
Jäger M, Westhoff B, Portier S, Leube B, Hardt K, Royer-Pokora B, Gossheger G, Krauspe R (2007) Clinical outcome and genotype in patients with hereditary multipleexostoses. J Orthop Res 25:1541–1551
Francannet C et al (2001) Genotype-phenotype correlation in hereditary multiple exostoses. J Med Genet 38:430–434
McCormick C, Duncan G, Goutsos KT, Tufaro F (2000) The putative tumor suppressors EXT1 and EXT2 form a stable complex that accumulates in the Golgi apparatus and catalyzes the synthesis of heparan sulfate. Proc Natl Acad Sci USA 97:668–673
Benoist-Lasselin C, de Margerie E, Gibbs L et al (2006) Defective chondrocyte proliferation and differentiation in osteochondromas of MHE patients. Bone 39:17–26
Fogel GR, McElfresh EC, Peterson HA, Wicklund PT (1984) Management of deformities of the forearm in multiple hereditary osteochondromas. J Bone Jt Surg Am 66:670–680
Schmale GA, Conrad EU 3rd, Raskind WH (1994) The natural history of hereditary multiple exostoses. J Bone Jt Surg Am 76:986–992
Wicklund CL, Pauli RM, Johnston D, Hecht JT (1995) Natural history study of hereditary multiple exostoses. Am J Med Genet 55:43–46
Cates HE, Burgess RC (1991) Incidence of brachydactyly and hand exostosis in hereditary multiple exostosis. J Hand Surg Am 16:127–132
Matsumoto Y, Matsumoto K, Irie F, Fukushi J, Stallcup WB, Yamaguchi Y (2010) Conditional ablation of the heparan sulfate-synthesizing enzyme Ext1 leads to dysregulation of bone morphogenic protein signaling and severe skeletal defects. J Biol Chem 285:19227–19234
Shu C, Smith SS, Little CB, Melrose J (2013) Comparative immunolocalisation of perlecan, heparan sulphate, fibroblast growth factor-18, and fibroblast growth factor receptor-3 and their prospective roles in chondrogenic and osteogenic development of the human foetal spine. Eur Spine J 22:1774–1784
Felix NA, Mazur JM, Loveless EA (2000) Acetabular dysplasia associated with hereditary multiple exostoses. A case report. J Bone Jt Surg Br 82:555–557
Mordenti M, Ferrari E, Pedrini E, Fabbri N, Campanacci L, Muselli M, Sangiorgi L (2013) Validation of a new multiple osteochondromas classification through Switching Neural Networks. Am J Med Genet A 161A:556–560
Morrissy RT, Goldsmith GS, Hall EC, Kehl D, Cowie GH (1990) Measurement of Cobb angle on radiographs of patients who have scoliosis. Evaluation of intrinsic error. J Bone Jt Surg Am 72:320–327
King HA, Moe JH, Bradford DS et al (1983) The selection of fusion levels in thoracic idiopathic scoliosis. J Bone Jt Surg Am 65:1302–1313
Nash CL Jr, Moe JH (1969) A study of vertebral rotation. J Bone Jt Surg Am 51:223–229
Chen S, Zhao L, Roffey DM, Phan P, Wai EK (2014) Association of rs11190870 near LBX1 with adolescent idiopathic scoliosis in East Asians: a systematic review and meta-analysis. Spine J 14:2968–2975
Settle SH Jr, Rountree RB, Sinha A, Thacker A, Higgins K, Kingsley DM (2003) Multiple joint and skeletal patterning defects caused by single and double mutations in the mouse Gdf6 and Gdf5 genes. Dev Biol 254:116–130
Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P (1996) Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell 84:911–921
Matsuo I, Kimura-Yoshida C (2013) Extracellular modulation of Fibroblast Growth Factor signaling through heparan sulfate proteoglycans in mammalian development. Curr Opin Genet Dev 23:399–407
Anower-E-Khuda MF, Matsumoto K, Habuchi H et al (2013) Glycosaminoglycans in the blood of hereditary multiple exostoses patients: half reduction of heparan sulfate to chondroitin sulfate ratio and the possible diagnostic application. Glycobiology 23:865–876
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (#23592192).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsumoto, Y., Matsumoto, K., Harimaya, K. et al. Scoliosis in patients with multiple hereditary exostoses. Eur Spine J 24, 1568–1573 (2015). https://doi.org/10.1007/s00586-015-3883-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-015-3883-4