Abstract
Purpose
The aim of this study was to evaluate the usefulness of diffusion tensor imaging (DTI) in the detection of cervical spinal cord integrity alterations in different stages of degenerative spine disease, as well as to compare DTI parameters with selected cervical spinal stenosis measurements.
Methods
One hundred and thirty-two symptomatic patients (mean age 53.58 years) with different stages of cervical spondylosis and twenty-five control subjects (mean age 45.78 years) were enrolled in the study. DTI was performed with a 1.5 T MR scanner. Three hundred and forty-nine spine segments from C2/C3 to C5/C6 were evaluated and divided into five groups according to the degree of spinal cord compression. The values of fractional anisotropy (FA) and apparent diffusion coefficient at each level were calculated and their correlations with the degree of stenosis were analyzed.
Results
FA values differed significantly (p < 0.0001) at all levels between the control group and patients with cervical degenerative disease, including subjects without spinal cord compression visible on plain MR images. A significant (p < 0.01) positive correlation between the mean FA values and anteroposterior diameter of the spinal canal as well as space available for the spinal cord index was demonstrated at all investigated levels.
Conclusion
DTI is capable of revealing impairment of the cervical spinal cord microstructure at the very early stage of degenerative spine disease, even prior to spinal cord compression visible on plain MR. Anteroposterior spinal canal diameter as well as space available for the cord index is well related to spinal cord tissue integrity defined by DTI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Degenerative cervical spine disease is one of the most common causes of spinal cord injury. It is a multi-structural disease entity, which may affect all parts of the disco-vertebral unit, leading to the progressive narrowing of the spinal canal. The most serious complication is spinal cord compression, resulting in neural tissue damage and a syndrome of spinal cord dysfunction known as cervical spondylotic myelopathy (CSM). Magnetic resonance imaging (MRI) is the modality of choice for quantifying the extent of degenerative alterations and spinal canal narrowing, though it has been proven to have low sensitivity in myelopathy detection, ranging 15–65 % [1–6]. Abnormal T2 signal intensity of the compressed spinal cord, which is considered to reflect myelopathic changes, is not always present, even in very advanced stages of clinically evident myelopathy, and poorly correlates with disease severity [7, 8]. Also its significance in predicting the course of CSM in individual patients is unclear, although some authors found increased T2 signal intensity as a poor prognostic factor for surgical decompression [9, 10].
Cervical spondylotic myelopathy usually develops gradually, at the beginning in the mechanism of repeated injuries to the spinal cord during movements, later due to chronic neural and vascular compression [11]. It can be assumed that minor spinal cord damage occurs already at the early stage of this process, which clearly cannot be detected by conventional MR examination.
Diffusion tensor imaging (DTI) is a unique MR technique which allows for in vivo evaluation of water molecule movement in highly organized tissues, such as white matter and muscles, providing information about strength of water diffusion (measured by apparent diffusion coefficient, ADC), as well as the main direction and the degree of directionality of diffusion (measured by fractional anisotropy, FA) [12]. Therefore, DTI seems to be sensitive to pathological processes altering water diffusion at a microscopic level, far beyond the resolution of conventional MR imaging [13]. Several investigators have carried out DTI studies in patients suffering from CSM [1, 6–8, 13–15]. They found significant differences in FA and ADC values between patients and normal volunteers, even in the absence of T2 hyperintensity of the spinal cord. Almost all of these studies were performed on patients with symptomatic myelopathy and at an advanced stage of spinal cord compression on plain MRI.
The purpose of this study was to evaluate DTI utility in detecting changes in the cervical spinal cord microstructure, especially in the early stage of DSD and to assess correlation of typical cervical spinal stenosis measurements, such as anteroposterior spinal canal diameter (APSCaD), anteroposterior spinal cord diameter (APSCoD) and SAC (space available for spinal cord) values with diffusion tensor parameters: fractional anisotropy (FA) and apparent diffusion coefficient (ADC). To the best of our knowledge until now there has been only one study, which compared DTI parameters and spinal canal stenosis measurements [16].
Materials and methods
Patients and the control group
One hundred and thirty-two symptomatic patients (78 women and 54 men; age range 18–76 years, mean 53.58 years) with different stages of cervical spondylosis observed on plain MR images and 25 control subjects (14 women and 11 men; age range 27–80 years, mean age 45.78 years) were enrolled in the study. The inclusion criteria for the patients were clinical and radiological signs and symptoms of degenerative cervical spine disease. Patients with congenital spinal canal narrowing, central spinal canal widening, previous spinal surgery and with any incidental findings on plain MR images suggestive of neurological disorder that could bias the results (e.g. inflammatory changes) were excluded from the study.
All control subjects were asymptomatic volunteers with no history of neurological disorders, consisting mainly of the hospital staff.
The study was conducted in accordance with the guidelines of the local University Ethics Committee for conducting research involving humans. Each patient provided their signed informed consent to participate in the examination.
MR imaging and DTI protocol
The MR examinations were performed with a 1.5-T MR unit (Signa Hdx, GE Medical Systems) with 33mT/m maximum gradient strength, using a sixteen-channel coil dedicated for head and spine imaging. The MR protocol consisted of sagittal T1-weighted images (TR/TE 555/10 ms), sagittal and axial T2-weighted images (TR/TE 3,580/111 ms), sagittal T2-weighted FAT SAT images (TR/TE 3,380/118 ms), followed by axial DTI sequence.
DTI acquisition was based on single-shot spin-echo echo-planar imaging (SE/EPI) with the following parameters: TR/TE 10.000/99 ms, 160 × 160 mm field of view, matrix 96 × 96, in plane image resolution 1.6 × 1.6 mm with a 4-mm-thick axial slices parallel to the intervertebral disk space, no gap, number of acquisitions: 2. In each study, the examination frame was adjusted to cover the length of the spinal cord from the second to seventh cervical vertebrae. Diffusion was measured along 15 non-collinear directions with two b values: 0 and 1,000 mm2/s resulting in 1 image without and 14 with diffusion weighting. The acquisition time of DTI ranged from 5 to 7 min.
Image analysis
Plain MR images of the cervical spine of 132 patients and 25 control subjects were analyzed by two independent investigators (A.B. and J.B.). Each spine segment for each disc level from C2/C3 to C5/C6 was evaluated separately in terms of evidence of degenerative spine disease (DSD). A C6/C7 level was excluded from the analysis due to a large amount of artifacts occurring at this level on diffusion tensor (DTI) images. Three hundred and forty-nine spine segments were selected altogether (268 from the patients` group and 81 from the controls) and subsequently divided into five groups as follows (Table 1):
- A:
-
Control group with no signs of DSD
- B:
-
Compression of the dura with obliteration of the subarachnoid space
- C:
-
Spinal cord surface scalloping but without signs of compression
- D:
-
Spinal cord compression without any changes in signal intensity of the spinal cord
- E:
-
Marked spinal cord compression with hyperintense signal changes on T2-weighted images.
The number of groups representing different stages of stenosis (A–E) at investigated cervical spine levels is shown in Fig. 1. The level most commonly affected by degenerative changes was C5/C6, while the degenerative changes in the earliest stage were observed most often at C2/C3. As far as the control group is concerned, only spine levels without any spinal canal obliteration visible on plain MR images were included in the study, which gave the total number of 81 spinal cord segments.
The anteroposterior (AP) diameter of the spinal canal and of the spinal cord was measured on the axial T2-weighted images at each evaluated level. Space available for the cord (SAC) value was determined by subtracting the sagittal spinal cord diameter from the corresponding sagittal spinal canal diameter, according to the equation: SAC = APSCaD − APSCoD (space available for the cord = anteroposterior spinal canal diameter − anteroposterior spinal cord diameter).
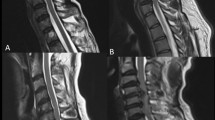
Image post-processing was done using the Functool software (GE ADW 4.4 workstation). Apparent diffusion coefficient (ADC) and fractional anisotropy (FA) transverse maps were generated and FA and ADC values were measured at the 268 levels corresponding to the selected spine segments presenting different stages of DSD, and at 81 reference spinal cord levels derived from healthy controls. The regions of interest (ROI) were drawn manually over the entire axial spinal cord cross section, according to the most accurate axial B0 image, as shown in Fig. 2. Special attention was paid to avoid partial volume effects, magnetic susceptibility effects and motion artifacts. In seven cases, ROI positioning was difficult to perform because of massive compression of the spinal cord (group E). These cases were not included in the statistical analysis.
Statistical analysis
Comparisons of the FA, ADC, AP spinal canal diameter (APSCaD), AP spinal cord diameter (APSCoD) and SAC values among all groups were made using a Student t test, ANOVA and LSD test. Comparisons were done between parameters derived from the same spinal cord levels. Correlations between spinal canal stenosis measurements (APSCaD, APSCoD and SAC values) and DTI parameters (FA, ADC) were estimated using Pearson’s correlation coefficients. Due to the small sample size, group E was excluded from statistical analysis. The Statistica10 software package was used for statistical calculations and p < 0.05 was considered statistically significant.
Results
The mean and standard deviations of FA, ADC, APSCaD, APSCoD and SAC values for four level segments (C2/C3–C5/C6) of the cervical spinal cord in control and patients’ groups are shown in Table 2. Table 3 shows comparison of the investigated parameters among groups.
DTI and the spinal canal stenosis measurements in the control group
The mean FA values in the control group ranged from 0.55 to 0.58 at different spinal cord level segments. The mean ADC values in the control group ranged from 0.77 to 0.86 × 10−3 mm2/s at different spinal cord level segments (Table 2).
The mean APSCaD, APSCoD and SAC values in the control group ranged as follows: from 13.67 to 15.05; from 7.42 to 8.16; from 6.04 to 6.89 respectively (Table 2).
Comparison of FA values between patients and control group and among patients` groups presenting different stages of DSD
FA values differed significantly (p < 0.01) at all four spinal cord levels between the control group and patients` groups presenting different stages of cervical DSD, including subjects without spinal cord compression or T2 hyperintensity on plain MR (Tables 2, 3).
Significant differences (p < 0.01) in the FA values were found between patients’ groups B and D as well as between groups C and D at all investigated levels.
Group C revealed lower FA values when compared to group B, but these changes were not statistically significant (p > 0.05) (Tables 2, 3).
Comparison of ADC values between patients and control groups and among patients` groups presenting different stages of DSD
The ADC values differed significantly (p < 0.05) between the control group and groups B and C at C3/C4–C5/C6 spine levels and between the control and D group at C5/C6 level (Tables 2, 3).
There were no statistically significant differences in ADC values among patients` groups (Tables 2, 3).
Correlations of FA and ADC values with the spinal canal stenosis measurements
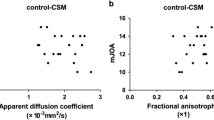
A significant positive correlation between the mean FA values and two parameters: APSCaD (r = 0.42, 0.64, 0.57, 0.51 respectively; p < 0.01) and SAC (r = 0.5, 0.59, 0.6, 0.57 respectively; p < 0.01) at levels C2/C3, C3/C4, C4/C5, C5/C6 was demonstrated.
There was a significant negative correlation between the mean ADC values and APSCaD values at C3/C4, C4/C5 and C5/C6 levels (r = −0.38, −0.27, −0.29 respectively; p < 0.01) (Table 4).
We found no statistically significant correlations between ADC values and APSCoD at all levels and between FA values and APSCoD in three of four investigated levels.
Discussion
MRI is considered the imaging modality of choice for the assessment of cervical spondylosis [17]; however, it is characterized by limited sensitivity in the evaluation of spinal cord involvement. So far, the only reliable way to assess intrinsic spinal cord structure changes has been via pathomorphological examination. The advent of DTI has made it possible to evaluate microstructural disturbances of the spinal cord in vivo [18, 19].
In the presented study, we investigated the usefulness of the DTI technique in the detection of cervical spinal cord structure alterations in different stages of DSD. We found statistically significant differences in FA and ADC values, between patients and controls, already at the stage of minor degenerative changes. There was a significant decrease in FA and increase in ADC values at C3/C4, C4/C5 and C5/C6 levels in the presence of only subarachnoid space obliteration, without any visible involvement of the spinal cord (group B).
To our knowledge, our study is the first one to show changes in main DTI metrics in cases without visible involvement of the spinal cord on plain MR. We hypothesize that the obtained results can be associated with dynamic factors, i.e., physiological motion of the spine. Muhle et al. [20], in the kinematic MRI study of patients with cervical spondylosis, reported that regardless of the stage of degenerative disease and the grade of spinal stenosis in the neutral position, cervical spinal motion may contribute to the development of CSM. During periods of movement, the spinal canal diameter is reduced about 2–3 mm on flexion [21] with the narrowing of the anterior space and anterior shifting of the cervical cord. In the majority of group B cases, it was anterior subarachnoid space to be narrowed, mainly due to disc herniation combined with initial osteophytic formation. Conversely, control group A was carefully selected so as no intervertebral disc reached beyond the borders of the adjacent vertebral bodies. Displacement of the spinal cord within the spinal canal, even without obvious spinal cord compression, may lead to abnormal tension patterns within the cord. It results in extracellular edema, which could cause the decrease in FA and increase in ADC values, as reported elsewhere in brain diseases [22]. There is an interesting hypothesis raised, among others, by Xiangshui et al., concerning the influence of increased pressure of cerebrospinal fluid on the internal pressure of the spinal cord. Cerebrospinal fluid flow disturbances, resulting from degenerative changes both in static position and during movement, could increase the pressure around the spinal cord and, in turn, alter interstitial diffusion which would result in DTI metrics changes [6].
The differences in FA and ADC values between control group and groups representing advanced, degenerative changes (D, E) were obvious, and has been already described by many authors [1, 6–8, 13–15].
In our study, the mean FA values in the control group ranged from 0.58 to 0.55 at different spinal cord level segments. There is a large variation in FA values of normal cervical spinal cord among different studies (0.55–0.745), which can be mainly explained by the different ROI setting methods used. From our data, it was impossible to distinguish between white and gray matter on b0 image and ROIs were drawn over the entire spinal cord cross section, which resulted in averaging FA and ADC values of white and gray matter. In our opinion, this method is relatively easy to use from a practical point of view, and could be reliably used for follow-up, provided that special attention during ROI setting is paid to avoid partial volume effect of an adjacent cerebrospinal fluid.
Regardless of the measurements method used, there is considerable variation in the absolute values of DTI parameters noticeable among healthy subjects which complicates the interpretation of DTI results in individual patients. Kerkovsky et al. [23], in a recent paper comparing diffusion tensor MR imaging with clinical findings, explored the usage of relative FA and ADC indices, which are the ratio of FA and ADC values derived from the unaffected segments (i.e. C2/C3 level) to the values obtained from the levels of spinal cord compression in one individual. This approach could help to avoid, among others, age-dependent divergences while comparing results and is certainly worth consideration.
There are several methods to quantitatively assess the reserve space around the spinal cord which is necessary to ensure adequate blood supply and biomechanics, and is usually obliterated in the course of DSD. We investigated two commonly used spinal canal measures: AP spinal canal diameter (APSCaD) and SAC value derived from transverse MRI images of both patients and controls. We found that the decrease of the mean FA values was significantly (p < 0.01) associated with the decrease of APSCaD and SAC value at all four spinal cord levels (C2/C3–C5/C6). Moreover, there was a significant (p < 0.01) negative correlation between the mean ADC values and APSCaD at three of four spine segments (C3/C4–C5/C6) as well as between mean ADC and SAC value (p < 0.01) at C3/C4 and C5/C6. These results suggest that anteroposterior spinal canal diameters as well as space available for the cord index are well related to spinal cord white matter integrity, and therefore, could be reliable indicators of spinal cord impairment in patients with degenerative cervical spine disease.
Conclusions
DTI provides clinically relevant information about spinal cord integrity and impairment that is not available from other imaging modalities. Diffusion tensor parameters, especially fractional anisotropy, are the most sensitive biomarkers of spinal cord tissue alterations. AP spinal canal diameter and SAC index, which in our study proved to have a good correlation with DTI indices, may indirectly provide information about spinal cord integrity in different stages of narrowing of the cervical spinal canal.
In the future, FA values could be the best tool for selecting patients for surgical intervention in patients with cervical myelopathy. The standardization of acquisition protocols and post-processing methods between the imaging centers is necessary for the widespread adoption of FA measurements in degenerative cervical spine disease.
References
Demir A, Ries M, Moonen CT et al (2003) Diffusion-weighted MR imaging with apparent diffusion coefficient and apparent diffusion tensor maps in cervical spondylotic myelopathy. Radiology 229:37–43
Matsuda Y, Miyazaki K, Tada K et al (1991) Increased MR signal intensity due to cervical myelopathy. Analysis of 29 surgical cases. J Neurosurg 74:887–892
Takahashi M, Yamashita Y, Sakamoto Y, Kojima R (1989) Chronic cervical cord compression: clinical significance of increased signal intensity on MR images. Radiology 173:219–224
Matsumoto M, Toyama Y, Ishikawa M et al (2000) Increased signal intensity of the spinal cord on magnetic resonance images in cervical compressive myelopathy. Does it predict the outcome of conservative treatment? Spine 25:677–682
Kang M, Anderer E, Elliott R, et al (2011) Diffusion tensor imaging of the spondylotic cervical spinal cord: a preliminary study of quantifiable markers in the evaluation for surgical decompression. Internet J Head Neck Surg http://ispub.com/IJHNS/5/1/6765
Xiangshui M, Xiangjun C, Xiaoming Z et al (2010) 3 T magnetic resonance diffusion tensor imaging and fiber tracking in cervical myelopathy. Clin Radiol 65:465–473. doi:10.1016/j.crad.2010.01.019
Wada E, Ohmura M, Yonenobu K (1995) Intramedullary changes of the spinal cord in cervical spondylotic myelopathy. Spine 20:2226–2232
Song T, Chen WJ, Yang B et al (2011) Diffusion tensor imaging in the cervical spinal cord. Eur Spine J 20:422–428. doi:10.1007/s00586-010-1587-3
Cho Y, Shin J, Kim K et al (2011) The relevance of intramedullary high signal intensity and gadolinium (Gd-DTPA) enhancement to the clinical outcome in cervical compressive myelopathy. Eur Spine J 20(12):2267–2274
Yukawa Y, Kato F, Yoshihara H et al (2007) MR T2 image classification in cervical compression myelopathy: predictor of surgical outcomes. Spine (Phila Pa 1976) 32:1675–1678 (discussion 1679)
Durrant DH, True JM (2002) Myelopathy, and radiculopathy and peripheral entrapment syndromes. CRC Press, Boca Raton
Sąsiadek MJ, Szewczyk P, Bladowska J (2012) Application of diffusion tensor imaging (DTI) in pathological changes of the spinal cord. Med Sci Monit 18:RA73–RA79
Budzik JF, Balbi V, Le Thuc V et al (2011) Diffusion tensor imaging and fibre tracking in cervical spondylotic myelopathy. Eur Radiol 21:426–433. doi:10.1007/s00330-010-1927
Lee JW, Kim JH, Park JB et al (2011) Diffusion tensor imaging and fiber tractography in cervical compressive myelopathy: preliminary results. Skeletal Radiol 40:1543–1551. doi:10.1007/s00256-011-1161
Kara B, Celik A, Karadereler S et al (2011) The role of DTI in early detection of cervical spondylotic myelopathy: a preliminary study with 3-T MRI. Neuroradiology 53:609–616. doi:10.1007/s00234-011-0844-4
Lindberg PG, Feydy A, Sanchez K et al (2012) Measures of spinal canal stenosis and relationship to spinal cord structure in patients with cervical spondylosis. J Neuroradiol 39:236–242. doi:10.1016/j.neurad.2011.09.004
Sasiadek MJ, Bladowska J (2012) Imaging of degenerative spine disease—the state of the art. Adv Clin Exp Med 21:133–142
Majumdar S, Zhu DC, Udpa SS et al (2011) A diffusion gradient optimization framework for spinal cord diffusion tensor imaging. Magn Reson Imaging 29:789–804. doi:10.1016/j.mri.2011.02.025
Van Hecke W, Leemans A, Sijbers J et al (2008) A tracking-based diffusion tensor imaging segmentation method for the detection of diffusion-related changes of the cervical spinal cord with aging. J Magn Reson Imaging 27:978–991. doi:10.1002/jmri.21338
Muhle C, Metzner J, Weinert D et al (1998) Classification system based on kinematic MR imaging in cervical spondylitic myelopathy. AJNR Am J Neuroradiol 19:1763–1771
Hochman M, Tuli S (2005) Cervical spondylotic myelopathy: a review. Internet J Neurol http://ispub.com/IJN/4/1/12262
Le Bihan D (1995) Diffusion and perfusion magnetic resonance imaging. Raven Press, New York, pp 50–57
Kerkovsky M, Bednarik J, Dusek L et al (2012) Magnetic resonance diffusion tensor imaging in patients with cervical spondylotic spinal cord compression: correlations between clinical and electrophysiological findings. Spine (Phila Pa 1976) 37:48–56
Acknowledgments
Supported by Wroclaw Medical University Grant ST-492.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Banaszek, A., Bladowska, J., Szewczyk, P. et al. Usefulness of diffusion tensor MR imaging in the assessment of intramedullary changes of the cervical spinal cord in different stages of degenerative spine disease. Eur Spine J 23, 1523–1530 (2014). https://doi.org/10.1007/s00586-014-3323-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-014-3323-x