Abstract
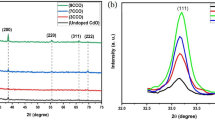
In this study, tungsten films with 150 and 300 nm thick were made by DC magnetron sputtering. The annealing treatment at 700 °C environment was used to characterize the influence of annealing duration (30, 45, 60, 75 and 90 min) on the morphology, phase and microstructure. Finally, we compared one-dimension W18O49 nanowires with one-dimension WO3 nanowires on NO2 gas sensing ability. In this research, W18O49 and WO3 were made as 150 nm thick films by annealing for 60 and 90 min at 700 °C environment, respectively. One-dimension W18O49 and WO3 nanowires were examined by X-ray diffraction (XRD). For the XRD result, the W18O49 with strongest diffraction peaks appears at 2θ = 23.454°, phase with (010) growth plan; while WO3 with strongest diffraction peaks appears at 2θ = 23.120°, 23.586°, 24.380°, phase with (002), (020), (200) growth plane. This study focused on the different NO2 concentrations (10, 20, 50 and 100 ppm) at a working temperature of 150 °C. W18O49 and WO3 gas sensing performance were dependent on NO2 concentrations. W18O49 sensing ability on different NO2 concentrations (10, 20, 50 and 100 ppm) was 1.436, 1.671, 1.816 and 1.973, respectively. WO3 sensing ability on different NO2 concentrations (10, 20, 50 and 100 ppm) was 1.462, 1.726, 1.970 and 2.365, respectively.


















Similar content being viewed by others

References
Akiyama M, Tamaki J, Miura N, Yamazoe N (1991) Tungsten oxide-based semiconductor sensor highly sensitive to NO and NO2. Chem Lett 20:1611–1614
An S, Park S, Ko H, Lee C (2014) Fabrication of WO3 nanotube sensors and their gas sensing properties. Ceram Int 40:1423–1429
Baratto C, Faglia G, Comini E, Sberveglieri G, Taroni A, La Ferrara V, Quercia L, Di Francia G (2001) A novel porous silicon sensor for detection of sub-ppm NO2 concentrations. Sens Actuators B Chem 77:62–66
Cao B, Chen J, Tang XJ, Zhou WL (2009) Growth of monoclinic WO3 nanowire array for highly sensitive NO2 detection. J Mater Chem 19:2323–2327
Choi HG, Jung YH, Kim DK (2005) Solvothermal synthesis of tungsten oxide nanorod/nanowire/nanosheet. J Am Ceram Soc 88:1684–1686
Heidari EK, Zamani C, Marzbanrad E, Raissi B, Nazarpour S (2010) WO3-based NO2 sensors fabricated through low frequency AC electrophoretic deposition. Sens Actuators B Chem 146:165–170
Hotovy I, Rehacek V, Siciliano P, Capone S, Spiess L (2002) Sensing characteristics of NiO thin films as NO2 gas sensor. Thin Solid Films 418:9–15
Hu M, Zeng J, Wang W, Chen H, Qin Y (2011) Porous WO3 from anodized sputtered tungsten thin films for NO2 detection. Appl Surf Sci 258:1062–1068
Hua ZQ, Wang Y, Wang HQ, Dong LA (2010) NO2 sensing properties of WO3 varistor-type gas sensor. Sens Actuators B Chem 150:588–593
Hung XJ, Choi YK (2007) Chemical sensors based on nanostructured materials. Sens Actuators B 122:659–671
Kim I-D, Rothschild A, Lee BH, Kim DY, Jo SM, Tuller HL (2006) Ultrasensi-tive chemiresistors based on electrospun TiO2 nanofibers. Nano Lett 6:2009–2013
Kim BG, Lim DG, Park JH, Choi YJ, Park JG (2011) In-situ bridging of SnO2 nanowires between the electrodes and their NO2 gas sensing characteristics. Appl Surf Sci 257:4715–4718
Kolmakov A, Moskovits M (2004) Chemical sensing and catalysis by one-dimensional metal-oxide nanostructures. Annu Rev Mater Res 34:151–180
Li GJ, Kawi S (1998) High-surface-area SnO2: a novel semiconductor-oxide gas sensor. Mater Lett 34:99–102
Li X, Lou T, Sun X, Li Y (2004) Highly sensitive WO3 hollow-sphere gas sensors. Inorg Chem 43:5442–5449
Miura N, Ono M, Shimanoe K, Yamazoe N (1998) A compact solid-state amperometric sensor for detection of NO2 in ppb range. Sens Actuators B Chem 49:101–109
Padilla-Rueda D, Vadillo JM, Laserna JJ (2012) Room temperature pulsed laserdeposited ZnO thin films as photoluminescence gas sensors. Appl Surf Sci 259:806–810
Polleux J, Gurlo A, Barsan N, Weimar U, Antonietti M, Niederberger M (2006) Template-free synthesis and assembly of single-crystalline tungsten oxide nanowires and their gas-sensing properties. Angew Chem Int Ed 45:261–265
Ponzoni A, Comini E, Sberveglieri G, Zhou J, Deng SZ, Xu NS, Ding Y, Wang ZL (2006) Ultrasensitive and highly selective gas sensors using three-dimensional tungsten oxide nanowire networks. Appl Phys Lett 88:203101–203103
Prasad AK, Kubinski DJ, Gouma PI (2003) Comparison of sol–gel and ion beam deposited MoO3 thin film gas sensors for selective ammonia detection. Sens Actuators B Chem 93:25–30
Qin Y, Hu M, Zhang J (2010) Microstructure characterization and NO2-sensing properties of tungsten oxide nanostructures. Sens Actuators B Chem 150:339–345
Qin Y, Liu C, Liu Y (2014) Nanowire (nanorod) arrays-constructed tungsten oxide hierarchical structure and its unique NO2-sensing performances. J Alloy Compd 615:616–623
Rout CS, Ganesh K, Govindaraj A, Rao CNR (2006) Sensors for the nitrogen oxides, NO2, NO and N2O, based on In2O3 and WO3 nanowires. Appl Phys A 85:241–246
Shen YB, Yamazaki T, Liu ZF, Meng D, Kikuta T, Nakatani N (2009) Influence of effective surface area on gas sensing properties of WO3 sputtered thin films. Thin Solid Films 517:2069–2072
Sun SB, Zou ZD, Min GH (2009) Synthesis of bundled tungsten oxide nanowires with controllable morphology. Mater Charact 60:437–440
Teoh LG, Hon YM, Shieh J, Lai WH, Hon MH (2003) Sensitivity properties of a novel NO2 gas sensor based on mesoporous WO3 thin film. Sens Actuators B Chem 96:219–225
Xia H, Wang Y, Kong F, Wang S, Zhu B, Guo X, Zhang J, Wang Y, Wu S (2008) Au-Doped WO3-based sensor for NO2 detection at low, operating temperature. Sens Actuators B 134:133–139
Zhang J, Liu X, Xu M, Guo X, Wu S, Zhang S, Wang S (2010) Pt clusters supported on WO3 for ethanol detection. Sens Actuators B 147:185–190
Zhou J, Ding Y, Deng SZ, Gong L, Xu NS, Wang ZL (2005) Three-dimensional tungsten oxide nanowire networks. Adv Mater 17:2107–2110
Zhu K, He H, Xie S, Zhang X, Zhou W, Jin S, Yue B (2003) Crystalline WO3 nanowires synthesized by templating method. Chem Phys Lett 377:317–321
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pan, C.T., Su, C.Y. & Luo, Y.C. Study on comparing WO3 and W18O49 gas sensing abilities under NO2 environment. Microsyst Technol 23, 2113–2123 (2017). https://doi.org/10.1007/s00542-016-3065-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00542-016-3065-2