Abstract
Purpose
The purpose of this study was to compare the effect of the long-term administration of flurbiprofen and fentanyl in the intensive care unit on natural killer cell cytotoxicity (NKCC), lymphocyte subsets and cytokine levels.
Methods
In this prospective study, patients scheduled for at least 48 h sedation after neck surgery were randomly assigned to two groups called group N and group F. Group N patients were sedated with propofol and flurbiprofen after surgery (n = 12), while group F patients were sedated with propofol and fentanyl (n = 13). The NKCC, lymphocyte subsets, and plasma levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and IL-10 were measured before and at the end of surgery, on postoperative day (POD) 1 and POD2.
Results
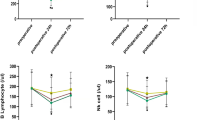
The NKCC was significantly higher on POD1 in group N than in group F (14.5 ± 11.2 versus 6.3 ± 4.1 %, p < 0.05), the difference between the groups disappearing on POD2. Lymphocyte subsets and plasma levels of cytokines were not significantly different between the two groups during the study period.
Conclusions
Transient suppressive effects on NKCC were observed in the fentanyl group as compared to the flurbiprofen group. This suggests that when choosing postoperative analgesics, physicians should bear in mind the potential immunosuppressive effects of these agents in patients requiring prolonged sedation in the intensive care unit.

Similar content being viewed by others
References
Meakins JL. Surgeons, surgery, and immunomodulation. Arch Surg. 1991;126:494–8.
Slade MS, Simmons RL, Yunis E, Greenberg LJ. Immunodepression after major surgery in normal patients. Surgery (St. Louis). 1975;78:363–72.
Ogawa K, Hirai M, Katsube T, Murayama M, Hamaguchi K, Shimakawa T, Naritake Y, Hosokawa T, Kajiwara T. Suppression of cellular immunity by surgical stress. Surgery (St. Louis). 2000;127:329–36.
Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth. 2008;22:263–77.
Watling SM, Dasta JF, Seidl EC. Sedatives, analgesics, and paralytics in the ICU. Ann Pharmacother. 1997;31:148–53.
Dasta JF, Fuhrman TM, McCandles C. Patterns of prescribing and administering drugs for agitation and pain in patients in a surgical intensive care unit. Crit Care Med. 1994;22:974–80.
Eisenstein TK, Hillburger ME. Opioid modulation of immune responses: effects on phagocyte and lymphoid cell population. J Neuroimmunol. 1998;83:36–44.
Yeager MP, Colacchio TA, Yu CT, Hildebrandt L, Howell AL, Weiss J, Guyre PM. Morphine inhibits spontaneous and cytokine-enhanced natural killer cell cytotoxicity in volunteers. Anesthesiology. 1995;83:500–8.
Soliman HM, Melot C, Vincent JL. Sedative and analgesic practice in the intensive care unit: the results of a European survey. Br J Anaesth. 2001;87:186–92.
Kadoi Y, Hinohara H, Kunimoto F, Saito S, Goto F. Fentanyl-induced hemodynamic changes after esophagectomy or cardiac surgery. J Clin Anesth. 2005;17:598–603.
Shavit Y, Ben-Eliyahu S, Zeidel A, Beilin B. Effects of fentanyl on natural killer cell activity and on resistance to tumor metastasis in rats. Dose and timing study. Neuroimmunomodulation. 2004;11:255–60.
Beilin B, Shavit Y, Hart J, Mordashov B, Cohn S, Notti I, Bessler H. Effects of anesthesia based on large versus small doses of fentanyl on natural killer cell cytotoxicity in the perioperative period. Anesth Analg. 1996;82:492–7.
Hussain M, Javeed A, Ashraf M, Al-Zaubai N, Stewart A, Mukhtar MM. Non-steroidal anti-inflammatory drugs, tumour immunity and immunotherapy. Pharmacol Res. 2012;66:7–18.
Kress JP, Hall JB. Sedation in the mechanically ventilated patient. Crit Care Med. 2006;34:2541–6.
Payen JF, Bru O, Bosson JL, Lagrasta A, Novel E, Deschaux I, Lavagne P, Jacquot C. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med. 2001;29:2258–63.
Yokoyama M, Itano Y, Mizobuchi S, Nakatsuka H, Kaku R, Takashima T, Hirakawa M. The effects of epidural block on the distribution of lymphocyte subsets and natural killer cell activity in patients with and without pain. Anesth Analg. 2001;92:463–9.
Volk T, Schenk M, Voigt K, Tohtz S, Putzier M, Kox WJ. Postoperative epidural anesthesia preserves lymphocyte, but not monocyte, immune function after major spine surgery. Anesth Analg. 2004;98:1086–92.
Patel SB, Kress JP. Sedation and analgesia in the mechanically ventilated patient. Am J Respir Crit Care Med. 2012;185:486–97.
Sanders RD, Hussell T, Maze M. Sedation & immunomodulation. Crit Care Clin. 2009;25:551–70.
Carr DJ, Rogers TJ, Weber RJ. The relevance of opioids and opioid receptors on immunocompetence and immune homeostasis. Proc Soc Exp Biol Med. 1996;213:248–57.
Lysle DT, Conssons ME, Watts VJ, Bennett EH, Dykstra LA. Morphine-induced alterations of immune status: dose dependency, compartment specificity and antagonism by naltrexone. J Pharmacol Exp Ther. 1993;265:1071–8.
Yardeni IX, Beilin B, Mayburd E, Alcalay Y, Bessler H. Relationship between fentanyl dosage and immune function in the postoperative period. J Opioid Manag. 2008;4:27–33.
Jacobs R, Karst M, Scheinichen D, Bevilacqua C, Schneider U, Heine J, Schedlowski M, Schmidt RE. Effects of fentanyl on cellular immune functions in man. Int J Immunopharmacol. 1999;21:445–54.
Yeager MP, Procopio MA, DeLeo JA, Arruda JL, Hildebrandt L, Howell AL. Intravenous fentanyl increases natural killer cell cytotoxicity and circulating CD16+ lymphocytes in humans. Anesth Analg. 2002;94:94–9.
Schneemilch CE, Hachenberg T, Ansorge S, Ittenson A, Bank U. Effects of different anaesthetic agents on immune cell function in vitro. Eur J Anaesthesiol. 2005;22:616–23.
Inada T, Kubo K, Shingu K. Possible link between cyclooxygenase-inhibiting and antitumor properties of propofol. J Anesth. 2011;25:569–75.
Kundu N, Walser TC, Ma X, Fulton AM. Cyclooxygenase inhibitors modulate NK activities that control metastatic disease. Cancer Immunol Immunother. 2005;54:981–7.
Benish M, Bartal I, Goldfarb Y, Levi B, Avraham R, Raz A, Ben-Eliyahu S. Perioperative use of β-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor. Ann Surg Oncol. 2008;15:2042–52.
Bastami S, Norling C, Trinks C, Holmlund B, Walz TM, Ahlner J, Uppugunduri S. Inhibitory effect of opiates on LPS mediated release of TNF and IL-8. Acta Oncol. 2012. doi:10.3109/0284186X.2012.737932
Inaoka M, Kimishima M, Takahashi R, Shiohara T. Non-steroidal anti-inflammatory drugs selectively inhibit cytokine production by NK cells and gamma delta T cells. Exp Dermatol. 2006;15:981–90.
Nelson CJ, Dykstra LA, Lysle DT. Comparison of the time course of morphine’s analgesic and immunologic effects. Anesth Analg. 1997;85:620–6.
Melamed R, Bar-Yosef S, Shakhar G, Shakhar K, Ben-Eliyahu S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg. 2003;97:1331–9.
Cronin AJ, Aucutt-Walter NM, Budinetz T, Bonafide CP, DiVittore NA, Gordin V, Schuler HG, Bonneau RH. Low-dose remifentanil infusion does not impair natural killer cell function in healthy volunteers. Br J Anaesth. 2003;91:805–9.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Narahara, H., Kadoi, Y., Hinohara, H. et al. Comparative effects of flurbiprofen and fentanyl on natural killer cell cytotoxicity, lymphocyte subsets and cytokine concentrations in post-surgical intensive care unit patients: prospective, randomized study. J Anesth 27, 676–683 (2013). https://doi.org/10.1007/s00540-013-1597-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-013-1597-5