Abstract
Purpose
The mitochondrial membrane potential (ΔΨm) is an important factor for apoptosis, and it is produced by the proton electrochemical gradient (ΔµH+). Therefore, the intracellular proton concentration (pHin) is an important factor for modifying the ΔΨm. However, the effects of lidocaine on pHin are unclear. To investigate mitochondrial responses to lidocaine, therefore, we simultaneously measured pHin with ΔΨm, flavin adenine dinucleotide (FAD), and reduced form of nicotinamide adenine dinucleotide (NADH) fluorescence, and calculated the FAD/NADH ratio (redox ratio), the superoxide production in mitochondria.
Methods
Morphological change and early apoptosis were observed by annexin-V FITC staining under fluorescent microscope. The ratiometric fluorescent probe JC-1 and HPTS were used for the simultaneous measurements of ΔΨm with pHin in rat dorsal root ganglion (DRG) neurons. FAD and NADH autofluorescence were simultaneously measured, and the FAD/NADH fluorescence ratio (redox ratio) was calculated. The superoxide was measured by mitosox-red fluorescent probe for mitochondrial superoxide. Lidocaine was evaluated at 1, 5, and 10 mM.
Results
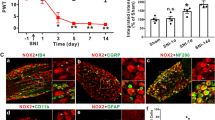
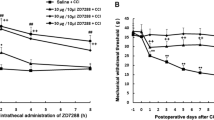
Morphological change and early apoptosis were observed after 10 mM lidocaine administration. Lidocaine depolarized ΔΨm with increased pHin in a dose-dependent manner. In low-pH saline (pH 6), in the presence of both the weak acids (acetate and propionate), lidocaine failed to depolarize ΔΨm and increase pHin. On the other hand, lidocaine decreased the redox ratio in the cell and increased the levels of superoxide in a dose-dependent manner.
Conclusion
These results demonstrated that lidocaine depolarizes ΔΨm by intracellular alkalization. These results may indicate one of the mechanisms responsible for lidocaine-induced neurotoxicity.







Similar content being viewed by others
References
Sakura S, Kirihara Y, Muguruma T, Kishimoto T, Saito Y. The comparative neurotoxicity of intrathecal lidocaine and bupivacaine in rats. Anesth Analg. 2005;101:541–7.
Takenami T, Yagishita S, Murase S, Hiruma H, Kawakami T, Hoka S. Neurotoxicity of intrathecally administered bupivacaine involves the posterior roots/posterior white matter and is milder than lidocaine in rats. Reg Anesth Pain Med. 2005;30:464–72.
Kasaba T, Onizuka S, Takasaki M. Procaine and mepivacaine have less toxicity in vitro than other clinically used local anesthetics. Anesth Analg. 2003;97:85–90.
Werdehausen R, Braun S, Essmann F, Schulze-Osthoff K, Walczak H, Lipfert P, Stevens MF. Lidocaine induces apoptosis via the mitochondrial pathway independently of death receptor signaling. Anesthesiology. 2007;107:136–43.
Johnson ME, Uhl CB, Spittler KH, Wang H, Gores GJ. Mitochondrial injury and caspase activation by the local anesthetic lidocaine. Anesthesiology. 2004;101:1184–94.
Tembe V, Henderson BR. BARD1 translocation to mitochondria correlates with Bax oligomerization, loss of mitochondrial membrane potential, and apoptosis. J Biol Chem. 2007;282:20513–22.
McGill A, Frank A, Emmett N, Turnbull DM, Birch-Machin MA, Reynolds NJ. The anti-psoriatic drug anthralin accumulates in keratinocyte mitochondria, dissipates mitochondrial membrane potential, and induces apoptosis through a pathway dependent on respiratory competent mitochondria. FASEB J. 2005;19:1012–4.
Andersson BS, Aw TY, Jones DP. Mitochondrial transmembrane potential and pH gradient during anoxia. Am J Physiol. 1987;252:349–55.
Kauppinen R. Proton electrochemical potential of the inner mitochondrial membrane in isolated perfused rat hearts, as measured by exogenous probes. Biochim Biophys Acta. 1983;725:131–7.
Broekemeier KM, Klocek CK, Pfeiffer DR. Proton selective substrate of the mitochondrial permeability transition pore: regulation by the redox state of the electron transport chain. Biochemistry. 1998;37:13059–65.
Marzulli D, La Piana G, Cafagno L, Fransvea E, Lofrumento NE. Proton translocation linked to the activity of the bi-trans-membrane electron transport chain. Arch Biochem Biophys. 1995;319:36–48.
Yokota K, Tatebayashi H, Matsuo T, Shoge T, Motomura H, Matsuno T, Fukuda A, Tashiro N. The effects of neuroleptics on the GABA-induced Cl– current in rat dorsal root ganglion neurons: differences between some neuroleptics. Br J Pharmacol. 2002;135:1547–55.
Liu T, Zhu W, Yang X, Chen L, Yang R, Hua Z, Li G. Detection of apoptosis based on the interaction between annexin V and phosphatidylserine. Anal Chem. 2009;81:2410–3.
Zhang G, Gurtu V, Kain SR, Yan G. Early detection of apoptosis using a fluorescent conjugate of annexin V. Biotechniques. 1997;23:525–31.
Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, Steele GD Jr, Chen LB. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci USA. 1991;88:3671–5.
Cossarizza A, Baccarani-Contri M, Kalashnikova G, Franceschi C. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1). Biochem Biophys Res Commun. 1993;197:40–5.
Willoughby D, Thomas RC, Schwiening CJ. Comparison of simultaneous pH measurements made with 8-hydroxypyrene-1,3,6-trisulphonic acid (HPTS) and pH-sensitive microelectrodes in snail neurons. Pflugers Arch. 1998;436:615–22.
Overly CC, Lee KD, Berthiaume E, Hollenbeck PJ. Quantitative measurement of intraorganelle pH in the endosomal–lysosomal pathway in neurons by using ratiometric imaging with pyranine. Proc Natl Acad Sci USA. 1995;92:3156–60.
Mokrý M, Gál P, Vidinský B, Kusnír J, Dubayová K, Mozes S, Sabo J. In vivo monitoring the changes of interstitial pH and FAD/NADH ratio by fluorescence spectroscopy in healing skin wounds. Photochem Photobiol. 2006;82:793–7.
Mukhopadhyay P, Rajesh M, Yoshihiro K, Haskó G, Pacher P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem Biophys Res Commun. 2007;358:203–8.
Jiang J, Serinkan BF, Tyurina YY, Borisenko GG, Mi Z, Robbins PD, Schroit AJ, Kagan VE. Peroxidation and externalization of phosphatidylserine associated with release of cytochrome c from mitochondria. Free Radic Biol Med. 2003;35:814–25.
Reutelingsperger CP, van Heerde WL. Annexin V the regulator of phosphatidylserine-catalyzed inflammation and coagulation during apoptosis. Cell Mol Life Sci. 1997;53:527–32.
Van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9.
Sanchez V, Arthur GR, Strichartz GR. Fundamental properties of local anesthetics I. The dependence of lidocaine’s ionization and octanol:buffer partitioning on solvent and temperature. Anesth Analg. 1987;66:159–65.
De Jong RH. Local anesthetic pharmacology. In: Brown DL, editor. Regional anesthesia and analgesia. Philadelphia: Saunders; 1996. p. 125–42.
Wilkie MP. Mechanisms of ammonia excretion across fish gills. Comp Biochem Physiol Part A Physiol. 1997;118:39–50.
John ET. The pharmacology of local anesthetics. Anesthesiol Clin N Am. 2000;18:217–33.
Mollica MP, Iossa S, Liverini G, Soboll S. Steady state changes in mitochondrial electrical potential and proton gradient in perfused liver from rats fed a high fat diet. Mol Cell Biochem. 1998;178:213–7.
Bernardi P, Vassanelli S, Veronese P, Colonna R, Szabó I, Zoratti M. Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations. J Biol Chem. 1992;267:2934–9.
Bernardi P. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by the proton electrochemical gradient. Evidence that the pore can be opened by membrane depolarization. J Biol Chem. 1992;267:8834–9.
Khaled AR, Reynolds DA, Young HA, Thompson CB, Muegge K, Durum SK. Interleukin-3 withdrawal induces an early increase in mitochondrial membrane potential unrelated to the Bcl-2 family. Roles of intracellular pH, ADP transport, and F(0)F(1)-ATPase. J Biol Chem. 2001;276:6453–62.
Adam-Vizi V. Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal. 2005;7:1140–9.
Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem. 2003;278:36027–31.
Fujimoto S, Nabe K, Takehiro M, Shimodahira M, Kajikawa M, Takeda T, Mukai E, Inagaki N, Seino Y. Impaired metabolism-secretion coupling in pancreatic beta-cells: role of determinants of mitochondrial ATP production. Diabetes Res Clin Pract. 2007;77:2–10.
Gutman M, Singer TP, Casida JE. Role of multiple binding sites in the inhibition of NADH oxidase by piericidin and rotenone. Biochem Biophys Res Commun. 1969;37:615–22.
Okun JG, Lümmen P, Brandt U. Three classes of inhibitors share a common binding domain in mitochondrial complex I (NADH:ubiquinone oxidoreductase). J Biol Chem. 1999;274:2625–30.
Irwin W, Fontaine E, Agnolucci L, Penzo D, Betto R, Bortolotto S, Reggiani C, Salviati G, Bernardi P. Bupivacaine myotoxicity is mediated by mitochondria. J Biol Chem. 2002;277:12221–7.
Zhuang J, Dinsdale D, Cohen GM. Apoptosis, in human monocytic THP.1 cells, results in the release of cytochrome c from mitochondria prior to their ultracondensation, formation of outer membrane discontinuities and reduction in inner membrane potential. Cell Death Differ. 1998;5:953–62.
Chen T, Wang J, Xing D, Chen WR. Spatio-temporal dynamic analysis of bid activation and apoptosis induced by alkaline condition in human lung adenocarcinoma cell. Cell Physiol Biochem. 2007;20:569–78.
Suzuki H, Yanaka A, Shibahara T, Matsui H, Nakahara A, Tanaka N, Muto H, Momoi T, Uchiyama Y. Ammonia-induced apoptosis is accelerated at higher pH in gastric surface mucous cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:986–95.
Acknowledgments
This work was supported in part by a Grant-in-Aid (No. 12770828) for Scientific Research (A) from The Ministry of Education, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Onizuka, S., Yonaha, T., Tamura, R. et al. Lidocaine depolarizes the mitochondrial membrane potential by intracellular alkalization in rat dorsal root ganglion neurons. J Anesth 25, 229–239 (2011). https://doi.org/10.1007/s00540-010-1079-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-010-1079-y