Abstract
Background
Adjuvant therapy may improve survival of patients with hepatocellular carcinoma (HCC) after curative resection. This study compared safety and efficacy outcomes between patients at high risk of recurrence who received different types of adjuvant therapy or no such therapy after hepatic resection for HCC.
Methods
Recurrence-free survival (RFS), overall survival, and adverse events were compared among patients who received adjuvant immune checkpoint inhibitors (ICIs) alone, ICIs with tyrosine kinase inhibitors (TKIs), or no adjuvant therapy between 13 March 2019 and 19 March 2022. This study was registered on ClinicalTrials.gov (NCT05221398).
Results
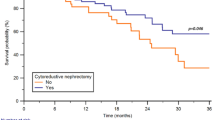
Of the 517 patients in final analysis, 432 (83.6%) received no adjuvant therapy, 53 (10.2%) received ICIs alone, and 32 (6.2%) received adjuvant ICIs and TKIs. During median follow-up of 34.0 months (IQR 27.8 to 41.6 months), RFS was significantly longer among patients who received either type of adjuvant therapy (25.2 months, 95%CI 16.4–34.0) than among those who received none (16.1 months, 95%CI 12.9–19.4), and this difference remained significant after propensity score matching (HR 0.52, 95%CI 0.35–0.76, P = 0.004). Overall survival was unaffected by either type of adjuvant therapy, while significant difference was observed between patients who received adjuvant therapy or not after propensity score matching (HR 0.31, 95%CI 0.17–0.59, P = 0.005). The rate of grade 3 or 4 adverse events was similar between the two types of adjuvant therapy.
Conclusions
ICIs alone or with TKIs may improve RFS of patients at high risk of HCC recurrence after curative resection.





Similar content being viewed by others
Data availability
Data available on request due to privacy/ethical restrictions.
References
Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76:681–93.
European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
Kudo M, Kawamura Y, Hasegawa K, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer. 2021;10:181–223.
Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 Edition). Liver Cancer. 2020;9:682–720.
Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC) Korea. KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol. 2022;2022(28):583–705.
Gordan JD, Kennedy EB, Abou-Alfa GK, et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J Clin Oncol. 2020;38:4317–45.
Jimenez Exposito MJ, Akce M, Montero Alvarez JL, et al. CA209–9DX: phase III, randomized, double-blind study of adjuvant nivolumab vs placebo for patients with hepatocellular carcinoma (HCC) at high risk of recurrence after curative resection or ablation. Ann Oncol. 2018;29:viii267–8.
Vogel A, Zhu AX, Cheng AL, et al. 1017TiP KEYNOTE-937 trial in progress: adjuvant pembrolizumab in patients with hepatocellular carcinoma (HCC) and complete radiologic response after surgical resection or local ablation. Ann Oncol. 2020;31:S703.
Hack SP, Spahn J, Chen M, et al. IMbrave 050: a Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol. 2020;16:975–89.
Knox J, Cheng A, Cleary S, et al. A phase 3 study of durvalumab with or without bevacizumab as adjuvant therapy in patients with hepatocellular carcinoma at high risk of recurrence after curative hepatic resection or ablation: EMERALD-2. Ann Oncol. 2019;30:iv59–60.
Yuan BH, Li RH, Yuan WP, et al. Perioperative entecavir for patients with HBV-related hepatocellular carcinoma and low levels of viral DNA: analysis using propensity score matching. Oncotarget. 2017;8:51810–6.
Zhong JH, Ke Y, Zhu SL, et al. Adefovir dipivoxil is less expensive than lamivudine and associated with similar prognosis in patients with hepatitis B virus-related hepatocellular carcinoma after radical resection. Onco Targets Ther. 2016;9:6897–907.
Zhong JH, Li LQ. Postoperative adjuvant transarterial chemoembolization for participants with hepatocellular carcinoma: a meta-analysis. Hepatol Res. 2010;40:943–53.
Qi YP, Zhong JH, Liang ZY, et al. Adjuvant transarterial chemoembolization for patients with hepatocellular carcinoma involving microvascular invasion. Am J Surg. 2019;217:739–44.
Wu MS, Zhong JH, Chen K, et al. Association of CK19 expression with the efficacy of adjuvant transarterial chemoembolization after hepatic resection in hepatocellular carcinoma patients at high risk of recurrence. J Clin Transl Res. 2022;8:71–9.
Chen K, Luo CP, Ge DX, et al. Case report: Conversion therapy to permit resection of initially unresectable hepatocellular carcinoma. Front Oncol. 2022;12: 946693.
Chen K, Wei W, Liu L, et al. Lenvatinib with or without immune checkpoint inhibitors for patients with unresectable hepatocellular carcinoma in real-world clinical practice. Cancer Immunol Immunother. 2022;71:1063–74.
National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50 Accessed 3 May 2022.
Schneider BJ, Naidoo J, Santomasso BD, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39:4073–126.
Zhong JH, Xing BC, Zhang WG, et al. Repeat hepatic resection versus radiofrequency ablation for recurrent hepatocellular carcinoma: retrospective multicentre study. Br J Surg. 2022;109(1):71–8.
Liu L, Xie S, Teng YX, et al. Outcomes of liver resection for metabolic dysfunction-associated fatty liver disease or chronic hepatitis B-related HCC. Front Oncol. 2021;11: 783339.
Huo RR, Liu HT, Deng ZJ, et al. Dose-response between serum prealbumin and all-cause mortality after hepatectomy in patients with hepatocellular carcinoma. Front Oncol. 2020;10: 596691.
Li MJ, Xie S, Teng YX, et al. Comparison of survival rates as predicted by total tumor volume or tumor burden score in patients with hepatocellular carcinoma concurrent with fatty liver disease and hepatitis B virus. Expert Rev Gastroenterol Hepatol. 2023;17:499–507.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894–905.
Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2–3 study. Lancet Oncol. 2021;22:977–90.
Yang X, Sun H, Hu B, et al. 944P Adjuvant camrelizumab combined with apatinib treatment after resection of hepatocellular carcinoma in CNLC II and III stage: a single-center prospective phase II trial. Ann Oncol. 2021;32:S824.
Bai X, Chen Y, Liang T. Preliminary data of a prospective study on the safety and efficacy of donafenib combined with anti-PD-1 antibody as adjuvant therapy for patients with hepatocellular carcinoma (HCC). J Clin Oncol. 2022;40: e16131.
Kudo M, Ueshima K, Nakahira S, et al. Adjuvant nivolumab for hepatocellular carcinoma (HCC) after surgical resection (SR) or radiofrequency ablation (RFA) (NIVOLVE): a phase 2 prospective multicenter single-arm trial and exploratory biomarker analysis. J Clin Oncol. 2021;39:4070.
Chow P, Chen M, Cheng AL, et al. IMbrave050: Phase 3 study of adjuvant atezolizumab + bevacizumab versus active surveillance in patients with hepatocellular carcinoma (HCC) at high risk of disease recurrence following resection or ablation. Presented at: 2023 AACR Annual Meeting; April 14–19, 2023; Orlando, Florida. Abstract CT003.
Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–54.
Lee JH, Lee JH, Lim YS, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148(1383–1391): e6.
Mo HY, Liao YY, You XM, et al. Timely meta-analysis on the efficacy of adoptive immunotherapy for hepatocellular carcinoma patients after curative therapy. PLoS ONE. 2017;12: e0174222.
Huinen ZR, Huijbers EJM, van Beijnum JR, et al. Anti-angiogenic agents - overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat Rev Clin Oncol. 2021;18:527–40.
Pinato DJ, Fessas P, Cortellini A, et al. Combined PD-1/VEGFR blockade: a new era of treatment for hepatocellular cancer. Clin Cancer Res. 2021;27:908–10.
Zhou J, Sun HC, Huang ZY, et al. Adjuvant lenvatinib after radical resection in patients with hepatocellular carcinoma (HCC): Preliminary analysis of a prospective, multi-center, single-arm study. J Clin Oncol. 2022;40:E16158.
Chen J, Lu L, Wen TF, et al. 945P Adjuvant lenvatinib in combination with TACE for hepatocellular carcinoma patients with high risk of postoperative relapse (LANCE): updated results from a multi-center prospective cohort study. Ann Oncol. 2021;32:S824–5.
Li L, Liu HT, Teng YX, et al. Second-line treatment options for hepatocellular carcinoma: current state and challenges for the future. Expert Opin Investig Drugs. 2022;31:1151–67.
Pfister D, Nunez NG, Pinyol R, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:450–6.
Teng YX, Guo PP, Qin KZ, et al. Lenvatinib with or without immune checkpoint inhibitors in subsets of advanced hepatocellular carcinoma. EJMO. 2022;6:25–9.
Rooney MS, Shukla SA, Wu CJ, et al. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61.
Dudek M, Pfister D, Donakonda S, et al. Auto-aggressive CXCR6(+) CD8 T cells cause liver immune pathology in NASH. Nature. 2021;592:444–9.
Funding
This work was supported by the Specific Research Project of Guangxi for Research Bases and Talents (GuiKe AD22035057), the National Natural Science Foundation of China (82060510 and 82260569), the Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor (Guangxi Medical University), Ministry of Education (GKE-ZZ202217), the Self-raised Scientific Research Fund of the Ministry of Health of Guangxi Province (Z20200923 and Z20191054), and Guangxi Undergraduate Training Program for Innovation and Entrepreneurship (S202310598205).
Author information
Authors and Affiliations
Contributions
J-HZ conceived the study; all authors participated in the acquisition of the data; X-ML, LL, P-SW, Q-BS, and G-LZ performed follow-up of the data; LL, KC LM, and J-HZ analyzed the data; LL and J-HZ drafted and revised the manuscript; all authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
535_2023_2018_MOESM2_ESM.jpeg
Supplementary file2 Supplementary Figure 1: Albumin–bilirubin score in patients with or without adjuvant therapy at the time of recurrence (P=0.157) (JPEG 422 KB)
535_2023_2018_MOESM3_ESM.tif
Supplementary file3 Supplementary Figure 2: Forest plot of survival in patient subgroups after propensity score-matched, based on Cox regression. a, recurrence-free survival; b, overall survival. CI, confidence interval; HBsAg, hepatitis B virus surface antigen; HBV, hepatitis B virus (TIF 466 KB)
535_2023_2018_MOESM5_ESM.tif
Supplementary file5 Supplementary Figure 3: Comparison of survival between all patients who received ICIs alone or with TKIs. A, recurrence-free survival; B, overall survival. HR, hazard ratio; ICI, immune checkpoint inhibitor; TKI, tyrosine kinase inhibitor (TIF 403 KB)
535_2023_2018_MOESM6_ESM.tif
Supplementary file6 Supplementary Figure 4: Comparison of survival between all patients with or without fatty liver disease who received adjuvant therapy. A, recurrence-free survival; B, overall survival. HR, hazard ratio; FLD, fatty liver disease (TIF 369 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, L., Wu, PS., Liang, XM. et al. Adjuvant immune checkpoint inhibitors associated with higher recurrence-free survival in postoperative hepatocellular carcinoma (PREVENT): a prospective, multicentric cohort study. J Gastroenterol 58, 1043–1054 (2023). https://doi.org/10.1007/s00535-023-02018-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-023-02018-2