Abstract
Background
This study investigated time-course changes in skeletal muscle volume per year with tolvaptan in patients with refractory ascites that was unresponsive to loop diuretics and aldosterone antagonists.
Methods
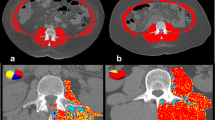
This retrospective study included 42 patients who received tolvaptan for refractory ascites and/or hepatic edema and underwent computed tomography (CT) before and ≥ 3 months after initiating tolvaptan. The time-course changes in skeletal muscle index per year [ΔSMI (%)] was calculated as follows: ΔSMI (%) = (SMI at final CT scan − SMI at initial CT scan)/SMI at initial CT scan × 100/years between CT scans.
Results
Eligible patients were 23 men and 19 women of median age of 71 years (range 21–94 years). The median follow-up period was 22.7 (range 3.5–54.6) months. ΔSMI (%) was significantly higher in the responders group than in the nonresponder group. Multivariate analysis showed the response to tolvaptan was an independent and significant factor associated with an increase in muscle mass [odds ratio (OR) 20.364; 95% CI 2.327–178.97; P = 0.006]. Overall survival with tolvaptan was significantly higher in the responder group than in the nonresponder group. Multivariate analysis showed that the response to tolvaptan treatment was a significant contributor to good prognosis (OR 3.884; 95% CI 1.264–11.931; P = 0.018). A significant negative correlation was observed between the dosage of furosemide and ΔSMI (%) (P = 0.014).
Conclusions
Treatment of refractory ascites with tolvaptan may attenuate the progression of sarcopenia and improve the prognosis in patients with decompensated cirrhosis.




Similar content being viewed by others
References
Montano-Loza AJ, Meza-Junco J, Prado CM, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166–73.
Hanai T, Shiraki M, Nishimura K, et al. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. 2015;31:193–9.
Yamada Y, Schoeller DA, Nakamura E, et al. Extracellular water may mask actual muscle atrophy during aging. J Gerontol A Biol Sci Med Sci. 2010;65:510–6.
Montano-Loza AJ. Clinical relevance of sarcopenia in patients with cirrhosis. World J Gastroenterol. 2014;20:8061–71.
Román E, García-Galcerán C, Torrades T, et al. Effects of an exercise programme on functional capacity, body composition and risk of falls in patients with cirrhosis: a randomized clinical trial. PLoS ONE. 2016;24(11):e0151652.
Hiraoka A, Michitaka K, Kiguchi D, et al. Efficacy of branched-chain amino acid supplementation and walking exercise for preventing sarcopenia in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2017;29:1416–23.
Snyder PJ, Peachey H, Berlin JA, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2670–7.
Ohara M, Ogawa K, Suda G, et al. Carnitine suppresses loss of skeletal muscle mass in patients with liver cirrhosis. Hepatol Commun. 2018;2:906–18.
Hiramatsu Akira, Aikata Hiroshi, Uchikawa Shinsuke, et al. Levocarnitine use is associated with improvement in sarcopenia in patients with liver cirrhosis. Hepatol Commun. 2019;3:345–55.
Dasarathy S. Consilience in sarcopenia of cirrhosis. J Cachexia Sarcopenia Muscle. 2012;3:225–37.
Sinclair M, Grossmann M, Hoermann R, et al. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: a randomised controlled trial. J Hepatol. 2016;65:906–13.
Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65:1232–44.
Qiu J, Thapaliya S, Runkana A, et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism. Proc Natl Acad Sci USA. 2013;110:18162–7.
Qiu J, Tsien C, Thapalaya S, et al. Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. Am J Physiol Endocrinol Metab. 2012;303:983–93.
Allard JP, Chau J, Sandokji K, et al. Effects of ascites resolution after successful TIPS on nutrition in cirrhotic patients with refractory ascites. Am J Gastroenterol. 2001;96:2442–7.
Kaido T, Uemoto S. Direct segmental multi-frequency bioelectrical impedance analysis is useful to evaluate sarcopenia. Am J Transpl. 2013;13:2506–7.
Fukui H, Saito H, Ueno Y, et al. Evidence-based clinical practice guidelines for liver cirrhosis 2015. J Gastroenterol. 2016;51:629–50.
Yamamura Y, Nakamura S, Itoh S, et al. OPC-41061, a highly potent human vasopressin V2-receptor antagonist: pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J Pharmacol Exp Ther. 1998;287:860–7.
Mitsiopoulos N, Baumgartner RN, Heymsfield SB, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–22.
Nishikawa H, Shiraki M, Hiramatsu A, et al. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res. 2016;46:951–63.
Hiramine Y, Uojima H, Nakanishi H, et al. Response criteria of tolvaptan for the treatment of hepatic edema. J Gastroenterol. 2018;53:258–68.
Witte MH, Witte CL, Dumont AE. Progress in liver disease: physiological factors involved in the causation of cirrhotic ascites. Gastroenterology. 1971;61:7420750.
Lieberman FL, Ito S, Reynolds TB. Effective plasma volume in cirrhosis with ascites. Evidence that a decreased value dose not account for renal sodium retention, a spontaneous reduction in glomerular filtration rate (GFR), and a fall in GFR during drug-induced diuretics. J Clin Inverst. 1969;48:975–81.
Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151–7.
Sakaida I, Kawasoe S, Kajimura K, et al. Tolvaptan for improvement of hepatic edema: a phase 3, multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Res. 2014;44:73–82.
Sakaida I, Terai S, Kurosaki M, et al. Effectiveness and safety of tolvaptan in liver cirrhosis patients with edema: interim results of post-marketing surveillance of tolvaptan in liver cirrhosis (START study). Hepatol Res. 2017;47:1137–46.
Gassanov N, Semmo N, Semmo M, et al. Vasopressin (AVP) and treatment with arginine vasopressin receptor antagonists (vaptans) in congestive heart failure, liver cirrhosis and syndrome of inappropriate antidiuretic hormone secretion (SIADH). Eur J Clin Pharmacol. 2011;67:333–46.
Decaux G, Soupart A, Vassart G. Non-peptide arginine-vasopressin antagonists; the vaptans. Lancet. 2008;371:1624–32.
Yi JH, Shin HJ, Kim HJ. V2 receptor antagonist: tolvaptan. Electrolyte Blood Press. 2011;9:50–4.
Sakaida I, Nakajima K, Okita K, et al. Can serum albumin level affect the pharmacological action of tolvaptan in patients with liver cirrhosis? A post hoc analysis of previous clinical trials in Japan. J Gastroenterol. 2015;50:1047–53.
Iwamoto T, Sakaida I, et al. Predictors of the effect of tolvaptan on the prognosis of cirrhosis. Intern Med. 2016;55:2911–6.
Kogiso T, Yamamoto K, Kobayashi M, et al. Response to tolvaptan and its effect on prognosis in cirrhosis. Hepatol Res. 2017;47:835–44.
Atsukawa M, Tsubota A, Kato K, et al. Analysis of factors predicting the response to tolvaptan in patients with liver cirrhosis and hepatic edema. J Gastroenterol Hepatol. 2018;33:1256–63.
Sakaida I, Terai S, Nakajima K, et al. Predictive factors of the pharmacological action of tolvaptan in patients with liver cirrhosis: a post hoc analysis. J Gastroenterol. 2017;52:229–36.
Nakagawa A, Atsukawa M, Tsubota A, et al. Usefulness of portal vein pressure for predicting the effects of tolvaptan in cirrhotic patients. World J Gastroenterol. 2016;22:5104–13.
Imamura T, Kinugawa K, Fujino T, et al. Increased urine aquaporin-2 relative to plasma arginine vasopressin is a novel marker of response to tolvaptan in patients with decompensated heart failure. Circ J. 2014;78:2240–9.
Nakanishi H, Kurosaki M, Hosokawa T, et al. Urinary excretion of the water channel aquaporin 2 correlated with the pharmacological effect of tolvaptan in cirrhotic patients with ascites. J Gastroenterol. 2016;51:620–7.
Hanai T, Shiraki M, Ohnishi S, et al. Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol Res. 2016;46:743–51.
Runyon BA, Committee APG. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087–107.
European Association for the Study of Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417.
Fede G, D’Amico G, Arvaniti V, et al. Renal failure and cirrhosis: a systematic review of mortality and prognosis. J Hepatol. 2012;56:810–8.
Mandai S, Furukawa S, Kodama M, et al. Loop diuretics affect skeletal myoblast differentiation and exercise-induced muscle hypertrophy. Sci Rep. 2017;7:46349.
Ishikawa, Naito S, Iimori S, et al. Loop diuretics are associated with greater risk of sarcopenia in patients with non-dialysis-dependent chronic kidney disease. PLoS One. 2018;13:e0192990.
Hanai T, Shiraki M, Miwa T, et al. Effect of loop diuretics on skeletal muscle depletion in patients with liver cirrhosis. Heptol Res. 2018. https://doi.org/10.1111/hepr.13244.
Freeman RB Jr, Wiesner RH, Harper A, et al. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8:851–8.
Kamath PS, Kim WR. The model for end-stage liver disease. Hepatology. 2007;45:797–805.
Tajika M, Kato M, Mohri H, et al. Prognostic value of energy metabolism in patients with viral liver cirrhosis. Nutrition. 2002;18:229–34.
Sam J, Nguyen GC. Protein-calorie malnutrition as a prognostic indicator of mortality among patients hospitalized with cirrhosis and portal hypertension. Liver Int. 2009;29:1396–402.
Meza-Junco J, Montano-Loza AJ, Baracos VE, et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol. 2013;47:861–70.
Kim WR, Biggins SW, Kremersetal WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–26.
Nishikawa H, Kita R, Kimura T, et al. Hyponatremia in hepatocellular carcinoma complicating with cirrhosis. J Cancer. 2015;6:482–9.
Ripoll C, Bañares R, Rincón D, et al. Influence of hepatic venous pressure gradient on the prediction of survival of patients with cirrhosis in the MELD era. Hepatology. 2005;42:793–801.
Planas R, Montoliu S, Balleste B, et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol. 2006;4:1385–94.
Cardenas A, Arroyo V. Management of ascites and hydrothorax. Best Pract Res Clin Gastroenterol. 2007;21:55–75.
Acknowledgements
This research is supported by AMED under Grant number JP19fk0210040.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kazuaki Chayama received honororira from Eizai, AbbVie, Gilead, Dainippon Sumitomo, Bristol-Myers Squibb, Mitsubishi Tanabe and Othuka, and research funding from Dainippon Sumitomo, The Chugoku Electric Power, Toray, EA Pharma, AbbVie, Eizai, Otsuka, MSD, Daiichi Sankyo, Takeda, Roche, Nippon Kayaku, Bristol-Myers Squibb and Mochida Phamaceutical.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Namba, M., Hiramatsu, A., Aikata, H. et al. Management of refractory ascites attenuates muscle mass reduction and improves survival in patients with decompensated cirrhosis. J Gastroenterol 55, 217–226 (2020). https://doi.org/10.1007/s00535-019-01623-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-019-01623-4