Abstract
Cirrhosis is the consequence of progression of many forms of necro-inflammatory disorders of the liver with hepatic fibrosis, hepatocellular dysfunction, and vascular remodeling. Reversing the primary hepatic disorder, liver transplantation, and controlling the complications are the major management goals. Since the former options are not available to the majority of cirrhotics, treating complications remains the mainstay of therapy. Sarcopenia and/or cachexia is the most common complication and adversely affects survival, quality of life, development of other complications of cirrhosis, and outcome after liver transplantation. With the increase in number of cirrhotic patients with hepatitis C and nonalcoholic fatty liver disease, the number of patients waiting for a liver transplantation is likely to continue to increase above the currently estimated 72.3/100,000 population. One of the critical clinical questions is to determine if we can treat sarcopenia of cirrhosis without transplantation. No effective therapies exist to treat sarcopenia because the mechanism(s) of sarcopenia in cirrhosis is as yet unknown. The reasons for this include the predominantly descriptive studies to date and the advances in our understanding of skeletal muscle biology and molecular regulation of atrophy and hypertrophy not being translated into the clinical practice of hepatology. Satellite cell biology, muscle autophagy and apoptosis, and molecular signaling abnormalities in the skeletal muscle of cirrhotics are also not known. Aging of the cirrhotic and transplanted population, use of mTOR inhibitors, and the lack of definitive outcome measures to define sarcopenia and cachexia in this population add to the difficulty in increasing our understanding of hepatic sarcopenia/cachexia and developing treatment options. Recent data on the role of myostatin, AMP kinase, impaired mTOR signaling resulting in anabolic resistance in animal models, and the rapidly developing field of nutriceuticals as signaling molecules need to be evaluated in human cirrhotics. Finally, the benefits of exercise reported in other disease states with sarcopenia may not be safe in cirrhotics due to the risk of gastrointestinal variceal bleeding due to an increase in portal pressure. This article focuses on the problems facing both muscle biologists and hepatologists in developing a comprehensive approach to sarcopenia in cirrhosis.
Similar content being viewed by others
1 Introduction
The need for consilience or unity of knowledge from different fields is best exemplified in the field of sarcopenia of liver disease. Knowledge from hepatology, gerontology, nutrition, muscle biology, developmental biology, transplant surgery, epidemiology, molecular biology, physiology, and metabolism need to be integrated to improve our understanding of the mechanisms of loss of skeletal muscle mass in cirrhosis. Loss of skeletal muscle mass or sarcopenia is the most common complication of cirrhosis and adversely affects survival, quality of life, outcome after liver transplantation, and response to stress including infection and surgery [1]. Cachexia and sarcopenia are recognized as a major complication of a number of chronic diseases including cancer, chronic obstructive pulmonary disease, chronic heart failure, and chronic renal failure [1–4]. It is interesting that there has been little focus on the prevalence, impact, consequences, and relative paucity of mechanistic targets or therapy of sarcopenia and cachexia in cirrhosis, especially given that the liver is the most metabolically active organ that regulates and interacts with the skeletal muscle and adipose tissue to form a “metabolic organ complex” [5, 6]. Cirrhosis is a form of end-stage liver disease that is the culmination of a number of chronic liver disorders. Since there is no therapy to reverse the fibrosis, hepatocellular dysfunction and vascular remodeling in cirrhosis, management has focused on treating the etiology of the liver disease, prevention and therapy of the complications, and replacing the diseased liver by transplantation. Few effective therapeutic modalities exist to treat the etiology of the disease and this contributes to the increase in incidence of cirrhosis [7–9]. Clinically, the most widely recognized complications include ascites, encephalopathy, portal hypertension with variceal bleeding, renal dysfunction, and hepatocellular carcinoma; however, the most frequent complication is malnutrition comprised of sarcopenia or loss of muscle mass and loss of fat mass or a combination constituting “hepatic cachexia”. Prevention or management of complications of cirrhosis has been the mainstay of therapy in hepatology, but there has been inadequate focus on managing sarcopenia or cachexia in these patients [10]. This is reflected by the lack of a clear definition of the components of malnutrition, the largely descriptive but not mechanistic studies, and the consequent lack of clear therapeutic strategies [11]. Although, it is believed that liver transplantation is curative for cirrhosis, this therapeutic option does not exist for the majority of patients [12]. Therefore, nontransplant therapeutic options remain the primary management option with the need to focus on reduced muscle mass because of its clinical relevance.
2 Malnutrition is the most frequent clinical problem in cirrhosis
Data from the Centers for Disease Control in 2006 showed that the annual incidence of cirrhosis is 72.3 per 100,000 population with an annual mortality rate of 27,000 [13]. Additional data support similar prevalence rates of cirrhosis both in the Western hemisphere and globally [13–18]. With the assumption of the baseline prevalence of 0.15 %, the total number of cirrhotics in the USA in 2006 is estimated to be 2.5 million. An alternative database search also yielded similar data from the VA system [17, 19]. Data were available from 2000 to 2007 and are shown in Fig. 1 [19]. These estimates further confirm the data from the organ procurement and transplant network that the prevalence of cirrhosis is progressively increasing based on the new patients added to the active and inactive waiting lists. Given the background of these staggering numbers, it is important to underscore the fact that the increase in annual liver transplant rates (17.42 to 22.29/million population) has not kept pace with the increase in numbers of patients with cirrhosis (Scientific Registry of Transplant Recipients (SRTR): 2009 OPTN/SRTR Annual Report 1999-2008. HHS/HRSA/HSB/DOT). Using the highest liver transplantation rate of 22.29/million (in 2006), the number of nontransplanted cirrhotics would increase by about 14,000/year based on the US population assumption of 300 million, without taking into account the projected increases related to hepatitis C and nonalcoholic fatty liver disease [7, 8, 14]. Using these very conservative figures, the nontransplant management of cirrhosis will be required for over 2.5 million patients by the end of this decade. The goals of this approach are to increase transplant free survival, decrease the risks of complications, and improve quality of life especially since a reduced post transplant quality of life is being increasingly recognized [11, 20–22].
Annual number of patients diagnosed with cirrhosis in the VA system and the annual liver transplants done in the USA [17, 19]. If the total number of patients with cirrhosis diagnosed each year is assumed to follow the VA system, then the widening gap between these two graphs depicts the number of cirrhotics who are not transplanted and therefore need nontransplant therapeutic options. This gap has continued to widen over the past decade and is anticipated to follow this pattern till 2020. Data compiled from OPTN / SRTR 2009 Annual Data Report. HHS/HRSA/HSB/DOT. http://optn.transplant.hrsa.gov/ar2009/data_tables_section9.htm
Transplant free survival depends on the severity of the underlying liver disease as well as management of the complications of cirrhosis [23]. Malnutrition has been reported in 60–80 % of patients with cirrhosis, but when sarcopenia was specifically evaluated, it occurred in about 40 % of cirrhotics [24, 25]. Esophageal varices are present in 30–60 % of patients with cirrhosis. Refractory ascites occurs in 5–10 % of patients and spontaneous bacterial peritonitis occurs in about 30 % of patients with ascites [23]. Hepatorenal syndrome occurs in about 8 % of cirrhotic patients with ascites. Portopulmonary hypertension and hepatopulmonary syndrome occur in about 0.5–5 % of cirrhotics. The annual incidence of hepatocellular carcinoma is 1.4 % in compensated and 4 % in decompensated cirrhosis. Extensive studies have been published on these complications as of September 30, 2011 and the unfiltered numbers of human studies published and indexed in PUBMED are shown in Table 1. As can be seen, the number of publications on sarcopenia in cirrhosis is very small and if the search term is expanded to cachexia or malnutrition, the number of publications is higher but the studies are no longer specific to muscle loss.
3 Malnutrition and its specific components in cirrhosis
The major problems with the term “malnutrition in cirrhosis” is the lack of focus on the specific components that constitute malnutrition, i.e., sarcopenia, loss of visceral or subcutaneous adipose tissue mass, alteration in substrate utilization and disordered energy metabolism, and a combination to varying extent of these disorders. One definition for malnutrition that has been proposed is that it is “a state of nutrition in which a deficiency or excess (or imbalance) of energy, protein and other nutrient causes adverse effects on body composition, function and outcome” [26]. The limitation of this definition is that it lacks specifics when it comes to the definition or terminology, especially in the background of advanced liver disease. Recent attempts to arrive at a consensus definition for sarcopenia, cachexia, and precachexia have focused on nonhepatic diseases, aging, and cancers. However, these definitions and the underlying mechanisms may need to be modified in patients with cirrhosis.
A number of complex metabolic alterations occur in liver disease that are unique to cirrhosis and affect the skeletal muscle growth and atrophy responses. These include dysregulation of fatty acid oxidation and ketogenesis, gluconeogenesis from amino acids, glycogenolysis, and selective utilization of aromatic amino acids in the liver and branched chain amino acids in the skeletal muscle as a source of energy [27, 28]. A number of biochemical, cytokine, hormonal, and neurological abnormalities in advanced liver disease are also similar to those in other chronic disorders [29]. Therefore, it is essential to have a consensus definition for the terms precachexia, cachexia, and sarcopenia in cirrhotic patients that has common features with other chronic diseases and certain essential differences. Inclusion of patients with cirrhosis while such definitions are being generated will assist in both consilience and translation of the rapid advances occurring in the field of myology and nutrition into clinical application for these patients. Sarcopenia is characterized by loss of muscle mass and has been used to define the loss of muscle mass in aging even though it is now being used in other disease states. Cachexia is defined as loss of both fat and muscle mass [6, 30–32]. Additional terms that have been used include precachexia that is defined by the unintentional weight loss of <5 % of usual body weight in the last 6 months, in the background of an underlying chronic disease, while sarcopenic obesity is used to refer to the disproportionate loss of muscle mass in the presence of increased adipose tissue mass [5, 32]. Missing are clear generalizable definitions and establishment of normal values. Most publications use historical norms and younger subjects to define sarcopenia that may not necessarily reflect the patient population [33, 34]. The potential of historical controls having different growth patterns, adipose tissue, and muscle mass in adulthood needs to be addressed. Additionally, the large-scale changes in the population demographics, mobility, and ethnicity are likely to affect the normative values. Furthermore, a recent comment that one of the hallmarks of cachexia is that loss of lean body mass cannot be prevented or reversed by simply increasing nutritional intake is of critical importance in the management of cirrhotic patients since neither cachexia nor sarcopenia is clearly defined in the cirrhotic population, but they do suffer from either or both [35]. Given the absence of standardized terminology in patients with liver disease, there is a compelling need to define these terms taking into consideration, the metabolic abnormalities specific for cirrhosis. Finally, it must be reiterated that even though the signaling pathways responsible for regulation of skeletal muscle mass are altered, it is not clear if the same alterations occur in all chronic noncommunicable diseases [36, 37].
4 Clinical impact of sarcopenia, cachexia, and malnutrition in cirrhosis
Since liver transplantation is not available or necessary for the majority of cirrhotics, nontransplant options are required and the end points for the therapy of these patients need to be redefined to improve quality of life, prevent and treat complications, and potentially extend survival [12, 13, 21, 22]. Using the published prevalence data, the total number of cirrhotic patients with reduced muscle mass is about 1.25 million. Sarcopenia is the most common complication of cirrhosis with very limited data on the mechanisms responsible for it and few treatment options (24). It contributes to the aggravation of other complications of cirrhosis including encephalopathy, ascites, and portal hypertension [28, 38–43]. It is also the major contributor to impaired quality of life in these patients [44–47]. In contrast to the very limited literature on sarcopenia or muscle loss in liver disease [24, 25], there is an explosion of data on sarcopenia in aging and molecular mechanisms of regulation of muscle loss and re-growth [30, 48–54]. Unfortunately, these very interesting and exciting advances in the field of muscle biology have had very limited translation into human cirrhosis. Our recent preliminary data that an increase in muscle mass [55, 56] is likely to improve survival provides an impetus for rapid translation to clinical use of many of the novel concepts in skeletal muscle loss including the role of satellite cells, autophagy, myostatin regulation of muscle mass, proteolytic pathways, metabolic regulation of muscle protein kinetics and the molecular regulation and integration of protein synthesis, breakdown, and satellite cell-mediated regeneration [37, 57, 58].
5 Obese cirrhotic
An additional consideration in defining sarcopenia is the impact of weight loss and muscle loss in the obese cirrhotic [59–61]. While the traditional clinical profile of a patient with progressive worsening of liver disease is loss of muscle and adipose tissue mass, the clinical profile of patients with cirrhosis is changing with the rapid increase in the number patients with nonalcoholic fatty liver disease that occurs in the setting of obesity [59, 62]. The current focus on the management of the obese cirrhotic is to promote weight loss to prevent progression of the necro-inflammation and fibrosis of nonalcoholic fatty liver disease [63–65]. However, the benefit of weight loss in cirrhosis related to nonalcoholic fatty liver disease is as yet unknown [63]. This is of clinical significance because of recent suggestions that overweight patients may have a better survival with chronic diseases classically associated with cachexia including those with advanced cancer, end-stage heart failure, renal failure on hemodialysis, and aging [66, 67]. The term “reverse epidemiology” has been applied to this paradox that unlike in the general population, overweight and obesity may not necessarily be detrimental to the short-term outcome in such patients [67, 68]. There are no data on the impact of obesity or weight loss in obese cirrhotic patients. Furthermore, it is also believed that in obese patients, a small weight loss can mask higher loss of skeletal muscle mass. This is especially important, since the end point of most clinical interventions is loss of whole body weight or body mass index, neither of which is a good measure of whole body skeletal muscle mass.
6 Aging cirrhotic
The aging of the population is accompanied by older cirrhotics. In fact, a review of the SRTR data (Fig. 2) showed that the most rapidly increasing population of patients with cirrhosis is over the age of 50 years. It is well recognized that the skeletal muscle mass in humans increases till the age of 20, is stable between 20 and 50, and after the age of 50 years, there is approximately a 1 % loss of muscle mass [69, 70]. This rapidly increasing population of older cirrhotics waiting for transplantation is likely to suffer from the combined effects of aging and cirrhosis on reduced muscle mass and consequent complications and adverse outcomes. A significant proportion of patients remain on the transplant waitlist (OPTN / SRTR 2009 Annual Data Report. HHS/HRSA/HSB/DOT) for prolonged periods of time (Fig. 3) and this adds to the burden of cirrhotic.sarcopenia.
Numbers of patients with cirrhosis who are being placed on the liver transplant list annually and are active on the waiting list. Patients aged 50 years or more are forming the most rapidly increasing population of waitlisted patients on the transplant list. These patients are likely to have more sarcopenia due to the combined effects of aging and cirrhosis. Data compiled from OPTN / SRTR 2009 Annual Data Report. HHS/HRSA/HSB/DOT. http://optn.transplant.hrsa.gov/ar2009/data_tables_section9.htm
7 Post-transplant immunosuppressant effects
The most rapidly increasing immunosuppressive regimen being used for maintenance therapy after liver transplantation is noncalcineurin inhibitors like sirolimus and everolimus (Fig. 4). The impact of these mTOR inhibitors on muscle protein synthesis and autophagy is well known [71, 72]. However, their role in post-transplant skeletal muscle recovery has not been studied. Furthermore, their contribution to the post-liver transplant metabolic syndrome and development of sarcopenic obesity are also not known and need detailed human studies [73]. Additionally, calcineurin is required for skeletal muscle differentiation, hypertrophy, and fiber-type determination [74]. With the nearly universal use of calcineurin inhibitors after liver transplantation, the confounding effects of these on the reversal of pre-transplant sarcopenia is not known. It is therefore evident that post-transplantation immunomodulation is likely to affect the anticipated benefits after liver transplantation.
Waiting time on the active liver transplant list has not changed significantly over the past decade. Continued waiting increases the age of the patient and the severity of disease, both of which worsen sarcopenia and muscle function. Data compiled from OPTN / SRTR 2009 Annual Data Report. HHS/HRSA/HSB/DOT. http://optn.transplant.hrsa.gov/ar2009/data_tables_section9.htm
Use of mTOR and calcineurin inhibitors as maintenance immunosuppression after liver transplantation. This does not include the use of steroids alone which also contributes to sarcopenia. mTOR inhibitors result in impaired muscle protein synthesis as well as autophagy, both of which could aggravate sarcopenia after liver transplantation. Data compiled from OPTN / SRTR 2009 Annual Data Report. HHS/HRSA/HSB/DOT. http://optn.transplant.hrsa.gov/ar2009/data_tables_section9.htm
8 Potential mechanisms of sarcopenia and cachexia in cirrhosis
8.1 Metabolic studies show altered whole body protein turnover in cirrhosis
As mentioned earlier, studies to date have used isotopic tracer methodology and descriptive and correlation studies to explain the development of “malnutrition” in cirrhosis. Isotopic tracer studies have shown that whole body protein breakdown is increased, unaltered, or decreased [75–79]. Indirect measures of whole body protein synthesis have been either unaltered or decreased [77–80]. Additionally, rates of albumin synthesis that have been used in other chronic disorders are a poor measure of nutrient response since albumin is synthesized in the liver and has limited value as a measure of nutritional response to substrate administration [78].
8.2 Impact of complications of cirrhosis on protein turnover
Plasma concentrations of branched chain amino acids are reduced in cirrhosis and this may have a negative impact on skeletal muscle protein synthesis and breakdown [81, 82]. As mentioned earlier, complications of cirrhosis have an adverse impact on skeletal muscle mass and energy metabolism in cirrhosis. Following gastrointestinal bleeding, a large protein load deficient in isoleucine that results in enhanced amino acid oxidation, further reduction in plasma, and muscle amino acid pools contribute to the sarcopenia. This has major clinical implications with the potential use of isoleucine supplementation during gastrointestinal bleeding to prevent worsening muscle mass induced by gastrointestinal bleeding. Other complications including infection and encephalopathy also have the potential to aggravate skeletal muscle proteolysis and impaired protein synthesis in cirrhosis.
8.3 Etiology of cirrhosis and reduced muscle mass
Cirrhosis develops following chronic liver disease due to a number of etiologies that have been recognized to affect skeletal and fat mass. Among cirrhotics, the most severe loss of body protein, muscle area, and function has been reported in those with alcohol related and cholestatic diseases [83–85]. Alcohol has a direct adverse effect on skeletal muscle protein turnover and increased expression of myostatin [86]. Bile salts have been reported to increase muscle oxygen consumption and energy expenditure [87]. Since cirrhosis is a state of accelerated starvation, increased energy expenditure is likely to further impair protein synthesis and accelerate proteolysis.
8.4 Molecular mechanisms responsible for sarcopenia of cirrhosis
Data on the skeletal muscle signaling pathways regulating protein synthesis and breakdown have been identified but not studied in cirrhosis [37]. Satellite cell biology has been extensively evaluated and its contributory role in sarcopenia of aging identified [88]. However, there are no studies that have examined the role of impaired satellite cell function in human cirrhotic sarcopenia. A number of reasons contribute to this including the limited interest due to lack of availability of successful therapy, lack of focus on quality of life, and the belief that liver transplantation as a therapeutic advance will correct all the adverse consequences of cirrhosis. Additionally, the coagulopathy that is almost universal in advanced liver disease has been believed to preclude obtaining muscle biopsies safely. Encephalopathy and ascites limit performing complex and prolonged studies and the heterogeneity of the etiology and stage of the disease are additional factors that have resulted in limited advances in our understanding of the mechanisms of sarcopenia and cachexia in cirrhosis. Finally, given the cross-translational nature of the problem, it is difficult to identify investigators with established expertise in hepatology, metabolism, and molecular and skeletal muscle biology. Notwithstanding these limitations, some advances have been made in this field and lay the foundation for a greater intellectual interaction between hepatologists, developmental biologists, and those with expertise in muscle biology.
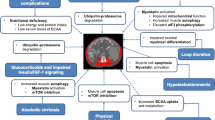
The past decade has seen enormous advances in understanding the mechanisms of sarcopenia of aging as well as the regulatory pathways that govern skeletal muscle atrophy, growth, regeneration, and satellite cell function. Discovery of myostatin, a TGFβ superfamily member, and the cross talk between the canonical insulin-like growth factor 1 (IGF1)–Akt–mTOR signaling as well as the ubiquitin proteasome pathways need to be examined in the context of cirrhosis [36, 37]. Identification of mTOR regulation by nutrient sensor, AMP kinase, general control of nutrition derepressed (GCN2) as a leucine sensor, and identification of increased skeletal muscle autophagy as a potential contributor to loss of muscle mass as well as its protective role during nutrient starvation are very exciting advances that have not been examined in a disease state [48, 49, 89]. Translation of the role of these signaling pathways in liver disease is likely to contribute to identification of potential therapeutic targets that is likely to result in the development of novel therapeutic measures to reverse sarcopenia and cachexia in cirrhosis.
In contrast to the sarcopenia of cirrhosis, other systemic disorders and aging are also accompanied by sarcopenia with consequent reduced survival and quality of life (Table 2) [70, 90–102]. The predominant mechanism(s) in these disorders has been an increased proteasome-mediated proteolysis with a lesser contribution from reduced protein synthesis. With aging, there is also a significant contribution of impaired satellite cell function. This has also been shown by us in an animal model with portosystemic shunting but not evaluated in other disease states.
Potential mechanisms of sarcopenia due to impaired protein synthesis in cirrhosis and targeted interventions. Data compiled from OPTN / SRTR 2009 Annual Data Report. HHS/HRSA/HSB/DOT. http://optn.transplant.hrsa.gov/ar2009/data_tables_section9.htm
9 Therapeutic options and outcome measures
Therapy for malnutrition in cirrhosis has focused on altering the hormonal and metabolic abnormalities of cirrhosis with very limited success. These have included growth hormone, insulin-like growth factor, testosterone, branched chain amino acid replacement, and late evening snack [103–105]. Quantifying the effectiveness of therapy requires precise outcome measures. Since skeletal muscle mass and strength have been related to survival and quality of life in cirrhosis as well as other chronic disorders with sarcopenia, studies that focus on such definite measures are necessary [24, 25, 83, 106, 107]. Once again, the outcome measures do not focus on reversal of sarcopenia, and limitations of the outcome measures for malnutrition in cirrhosis include the development of ascites and whole body volume overloading, sodium retention, changes in plasma and muscle amino acid concentrations, hyper- and hypometabolism in over 30 % of patients, and the use of lean body mass as a surrogate for skeletal muscle mass. Initial suggestions that low expression of skeletal muscle IGF1 was followed by therapeutic intervention studies using IGF1-IGF1 binding protein complex with limited benefit [103, 104, 108–110]. Recently, we have shown that an increased expression of skeletal muscle myostatin is responsible for reduced muscle protein synthesis and impaired satellite cells in a rat model of portosystemic shunting [56]. We have also shown that the adverse consequences of increased myostatin expression can be reversed without impacting the underlying liver disease which is especially exciting because of the aforementioned data that liver transplantation is not a universally available treatment option and reversing “hepatic cachexia–sarcopenia” can be a major therapeutic option for cirrhosis.
9.1 Nutriceuticals in sarcopenia of cirrhosis
The novel field of nutriceuticals has been shown to be effective in countering sarcopenia of aging [111–113]. These data have enormous application in patients with cirrhosis and systematic studies with defined outcome measures in reversal of sarcopenia and skeletal muscle function are needed. Use of leucine-enriched essential amino acids to stimulate mTOR independent of upstream inhibitors including myostatin and AMPK has been shown to be effective in other disorders with muscle atrophy [113–115]. Previous studies on branched amino acid supplementation in patients with cirrhosis have focused on their benefit in encephalopathy with nitrogen balance as a secondary outcome measure (Table 3) [116–123]. It is interesting that the focus of amino acid supplementation in cirrhosis is based on the lower plasma-branched chain amino acids suggesting that administering these amino acids would result in improvement in outcome. However, since sarcopenia in cirrhosis as in other conditions with anabolic resistance is believed to be due to impaired mTOR signaling response, it can potentially be overcome by leucine-enriched supplementation with amino acids [124–126]. When branched chain amino acids are administered, stimulation of protein synthesis by leucine is likely to result in increased utilization of other essential amino acids that may then become limiting for protein synthesis. Even though this has not been specifically evaluated in patients with cirrhosis, the alteration in plasma amino acid profile in response to branched chain versus balanced amino acids lends credence to this view [127]. This can be overcome by administering a balanced mixture of essential amino acids with leucine enrichment. Plasma concentrations of aromatic amino acids in cirrhosis are elevated and have been suggested to play a role in the development of hepatic encephalopathy [81]. Therefore, studies to determine the optimum concentration of appropriate amino acid supplementation to derive the maximum skeletal muscle protein response in cirrhotics are necessary. Furthermore, glutamine is a conditionally essential amino acid in cirrhosis and has been shown to directly suppress myostatin expression in muscle cells in vitro [128, 129]. The role of glutamine supplementation in addition to leucine may reverse the molecular abnormalities of increased myostatin and mTOR resistance in cirrhosis to maximize the beneficial effects. Extracellular rather than intracellular concentrations of amino acids have been shown to be potent regulators of muscle protein metabolic response [82]. Therefore, administration of the appropriate combination of essential amino acids to alter plasma concentrations can be an effective therapy in these patients [82, 130]. The integration of metabolic studies, molecular signaling pathway analyses, and clinically meaningful outcome measures are necessary to have a direct impact on the survival, complications, and quality of life in patients with cirrhosis. Few studies have examined the impact of interventions on skeletal muscle mass or strength in cirrhotics [131]. This is important because an increase in acute protein synthesis response does not always translate into an increase in muscle anabolism [53, 132]. Finally, the source of dietary proteins, the amino acid composition as well as the digestibility of different proteins and the age of the subject alter the muscle protein metabolic response [112, 133, 134].
9.2 Role of exercise in cirrhotic patients
The role of aerobic and resistance exercise on skeletal muscle insulin signaling, protein synthesis response, AMP kinase activity, and satellite cell function has been identified [50, 135]. However, fatigue, reduced maximum exercise capacity in cirrhotics, and the presence of complications including ascites, encephalopathy, and portal pressure have limited the translation of the data or the elegant designs of the studies performed in noncirrhotic patients [136–143]. Resistance exercise increases portal hypertension, and even transient increases in portal hypertension can result in catastrophic variceal bleeding and death [144]. It is therefore critical that the data on the impact of exercise on muscle mass and function be translated very judiciously in cirrhotic patients.
Novel strategies to reverse cachexia including myostatin antagonists are also of clinical interest especially given recent data that myostatin may play a critical role in cirrhotic sarcopenia [56, 145]. Given the paucity of data, the understudied nature of the problem, sarcopenia in cirrhosis deserves to be recognized as an area of unmet need with the potential to improve the outcome of the large number of patients with cirrhosis. One potential strategy for development of novel and successful therapies is the need for consilience between the diverse and seemingly unrelated fields identified earlier. A putative therapeutic approach to sarcopenia in cirrhosis is suggested in Fig. 5.
References
O'Brien A, Williams R. Nutrition in end-stage liver disease: principles and practice. Gastroenterology. 2008;134:1729–40.
Elkina Y, von Haehling S, Anker SD, Springer J. The role of myostatin in muscle wasting: an overview. J Cachexia Sarcopenia Muscle. 2011;2:143-151.
Kung T, Szabo T, Springer J, Doehner W, Anker SD, von Haehling S. Cachexia in heart disease: highlights from the ESC 2010. J Cachexia Sarcopenia Muscle. 2011;2:63-69.
Springer J, Adams V, Anker SD. Myostatin: regulator of muscle wasting in heart failure and treatment target for cardiac cachexia. Circulation. 2010;121:354–6.
Fearon K, Evans WJ, Anker SD. Myopenia—a new universal term for muscle wasting. J Cachexia Sarcopenia Muscle. 2011;2:1–3.
Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) "cachexia-anorexia in chronic wasting diseases" and "nutrition in geriatrics". Clin Nutr. 2010;29:154–9.
Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9:331–8.
Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–21. 521.
Deuffic-Burban S. Expected increase in prevalence of HCV-related cirrhosis and its complications in the United States: no effect of current antiviral treatment coverage? Gastroenterol Clin Biol. 2010;34:577–9.
Pinzani M, Rosselli M, Zuckermann M. Liver cirrhosis. Best Pract Res Clin Gastroenterol. 2011;25:281–90.
Kerwin AJ, Nussbaum MS. Adjuvant nutrition management of patients with liver failure, including transplant. Surg Clin North Am. 2011;91:565–78.
Cardenas A, Gines P. Management of patients with cirrhosis awaiting liver transplantation. Gut. 2011;60:412–21.
Lim YS, Kim WR. The global impact of hepatic fibrosis and end-stage liver disease. Clin Liver Dis. 2008;12:733–46. vii.
Bell BP, Manos MM, Zaman A, Terrault N, Thomas A, Navarro VJ, et al. The epidemiology of newly diagnosed chronic liver disease in gastroenterology practices in the United States: results from population-based surveillance. Am J Gastroenterol. 2008;103:2727–36.
Durante AJ, Meek JI, St LT, Navarro VJ, Sofair AN. Quantifying the burden of chronic viral hepatitis-related cirrhosis hospitalizations in New Haven County, Connecticut. Conn Med. 2008;72:393–7.
Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–38.
Public Health Strategic Health Care Group. Other diseases and conditions seen in veterans with chronic HCV State of Care Report IB 10-381. P96452. 2010. 15-21.
Vong S, Bell BP. Chronic liver disease mortality in the United States, 1990–1998. Hepatology. 2004;39:476–83.
Davison J, Indest D, Ross D. Addressing behavioral factors to improve liver health. Issue 4 ed. 2011. 2-4. http://www.publichealth.va.gov/docs/publichealth/public_health_matters_0309_new.pdf.
Braun F, Teren K, Wilms P, Gunther R, Allmann J, Broering DC, et al. Quality of life after liver transplantation. Transplant Proc. 2009;41:2564–6.
Elliott C, Frith J, Pairman J, Jones DE, Newton JL. Reduction in functional ability is significant postliver transplantation compared with matched liver disease and community dwelling controls. Transpl Int. 2011;24:588–95.
Grattagliano I, Ubaldi E, Bonfrate L, Portincasa P. Management of liver cirrhosis between primary care and specialists. World J Gastroenterol. 2011;17:2273–82.
Dong MH, Saab S. Complications of cirrhosis. Dis Mon. 2008;54:445–56.
Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, et al. Sarcopenia is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2012;10:166–73.
Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211:271–8.
Kinosian B, Jeejeebhoy KN. What is malnutrition? Does it matter? Nutrition. 1995;11:196–7.
Owen OE, Reichle FA, Mozzoli MA, Kreulen T, Patel MS, Elfenbein IB, et al. Hepatic, gut, and renal substrate flux rates in patients with hepatic cirrhosis. J Clin Invest. 1981;68:240–52.
Kondrup J, Nielsen K, Hamberg O. Nutritional therapy in patients with liver cirrhosis. Eur J Clin Nutr. 1992;46:239–46.
Dasarathy S. Inflammation and liver. JPEN J Parenter Enteral Nutr. 2008;32:660–6.
von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1:129-133.
Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12:403–9.
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95.
Frisancho AR. New standards of weight and body composition by frame size and height for assessment of nutritional status of adults and the elderly. Am J Clin Nutr. 1984;40:808–19.
Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700.
Lee SJ, Glass DJ. Treating cancer cachexia to treat cancer. Skelet Muscle. 2011;1:2.
Glass D, Roubenoff R. Recent advances in the biology and therapy of muscle wasting. Ann N Y Acad Sci. 2010;1211:25–36.
Ruegg MA, Glass DJ. Molecular mechanisms and treatment options for muscle wasting diseases. Annu Rev Pharmacol Toxicol. 2011;51:373–95.
Huisman EJ, Trip EJ, Siersema PD, van Hoek B, van Erpecum KJ. Protein energy malnutrition predicts complications in liver cirrhosis. Eur J Gastroenterol Hepatol. 2011;23:982-989.
Romiti A, Merli M, Martorano M, Parrilli G, Martino F, Riggio O, et al. Malabsorption and nutritional abnormalities in patients with liver cirrhosis. Ital J Gastroenterol. 1990;22:118–23.
Lata J, Husova L, Jurankova J, Senkyrik M, Dite P, Dastych Jr M, et al. Factors participating in the development and mortality of variceal bleeding in portal hypertension—possible effects of the kidney damage and malnutrition. Hepatogastroenterology. 2006;53:420–5.
Kalaitzakis E, Olsson R, Henfridsson P, Hugosson I, Bengtsson M, Jalan R, et al. Malnutrition and diabetes mellitus are related to hepatic encephalopathy in patients with liver cirrhosis. Liver Int. 2007;27:1194–201.
Kalaitzakis E, Bjornsson E. Hepatic encephalopathy in patients with liver cirrhosis: is there a role of malnutrition? World J Gastroenterol. 2008;14:3438–9.
Soros P, Bottcher J, Weissenborn K, Selberg O, Muller MJ. Malnutrition and hypermetabolism are not risk factors for the presence of hepatic encephalopathy: a cross-sectional study. J Gastroenterol Hepatol. 2008;23:606–10.
Larson AM, Curtis JR. Integrating palliative care for liver transplant candidates: "too well for transplant, too sick for life". JAMA. 2006;295:2168–76.
Mehta G, Rothstein KD. Health maintenance issues in cirrhosis. Med Clin North Am. 2009;901–15, viii-ix.
Norman K, Kirchner H, Lochs H, Pirlich M. Malnutrition affects quality of life in gastroenterology patients. World J Gastroenterol. 2006;12:3380–5.
Panagaria N, Varma K, Nijhawan S, Mathur A, Rai RR. Quality of life and nutritional status in alcohol addicts and patients with chronic liver disease. Trop Gastroenterol. 2007;28:171–5.
Dickinson JM, Fry CS, Drummond MJ, Gundermann DM, Walker DK, Glynn EL, et al. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr. 2011;141:856–62.
Drummond MJ, Bell JA, Fujita S, Dreyer HC, Glynn EL, Volpi E, et al. Amino acids are necessary for the insulin-induced activation of mTOR/S6K1 signaling and protein synthesis in healthy and insulin resistant human skeletal muscle. Clin Nutr. 2008;27:447–56.
Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, et al. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008;104:1452–61.
Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, et al. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587:1535–46.
Drummond MJ, Fry CS, Glynn EL, Timmerman KL, Dickinson JM, Walker DK, et al. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol. 2011;111:135–42.
Glynn EL, Fry CS, Drummond MJ, Timmerman KL, Dhanani S, Volpi E, et al. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr. 2010;140:1970–6.
Glynn EL, Fry CS, Drummond MJ, Dreyer HC, Dhanani S, Volpi E, et al. Muscle protein breakdown has a minor role in the protein anabolic response to essential amino acid and carbohydrate intake following resistance exercise. Am J Physiol Regul Integr Comp Physiol. 2010;299:R533–40.
Dasarathy S, Muc S, Runkana A, Mullen KD, Kaminsky-Russ K, McCullough AJ. Alteration in body composition in the portacaval anastamosis rat is mediated by increased expression of myostatin. Am J Physiol Gastrointest Liver Physiol. 2011;301:G731–8.
Dasarathy S, McCullough AJ, Muc S, Schneyer A, Bennett CD, Dodig M, et al. Sarcopenia associated with portosystemic shunting is reversed by follistatin. J Hepatol. 2011;54:915–21.
Ju JS, Varadhachary AS, Miller SE, Weihl CC. Quantitation of "autophagic flux" in mature skeletal muscle. Autophagy. 2010;6:929–35.
Paul PK, Gupta SK, Bhatnagar S, Panguluri SK, Darnay BG, Choi Y, et al. Targeted ablation of TRAF6 inhibits skeletal muscle wasting in mice. J Cell Biol. 2010;191:1395–411.
Hessheimer AJ, Forner A, Varela M, Bruix J. Metabolic risk factors are a major comorbidity in patients with cirrhosis independent of the presence of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2010;22:1239–44.
Mosko JD, Nguyen GC. Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:897–901.
Wree A, Kahraman A, Gerken G, Canbay A. Obesity affects the liver—the link between adipocytes and hepatocytes. Digestion. 2011;83:124–33.
Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–53.
Peng L, Wang J, Li F. Weight reduction for non-alcoholic fatty liver disease. Cochrane Database Syst Rev. 2011;(6):CD003619.
Cotrim HP, Freitas LA, Alves E, Almeida A, May DS, Caldwell S. Effects of light-to-moderate alcohol consumption on steatosis and steatohepatitis in severely obese patients. Eur J Gastroenterol Hepatol. 2009;21:969–72.
Tilg H, Moschen A. Weight loss: cornerstone in the treatment of non-alcoholic fatty liver disease. Minerva Gastroenterol Dietol. 2010;56:159–67.
Kalantar-Zadeh K, Horwich TB, Oreopoulos A, Kovesdy CP, Younessi H, Anker SD, et al. Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care. 2007;10:433–42.
Speakman JR, Westerterp KR. Reverse epidemiology, obesity and mortality in chronic kidney disease: modelling mortality expectations using energetics. Blood Purif. 2010;29:150–7.
Martin-Ponce E, Santolaria F, Aleman-Valls MR, Gonzalez-Reimers E, Martinez-Riera A, Rodriguez-Gaspar M, et al. Factors involved in the paradox of reverse epidemiology. Clin Nutr. 2010;29:501–6.
Berger MJ, Doherty TJ. Sarcopenia: prevalence, mechanisms, and functional consequences. Interdiscip Top Gerontol. 2010;37:94–114.
Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol. 2003;95:1717–27.
Machida M, Takeda K, Yokono H, Ikemune S, Taniguchi Y, Kiyosawa H, et al. Reduction of ribosome biogenesis with activation of the mTOR pathway in denervated atrophic muscle. J Cell Physiol. 2012;227:1569–76.
Sakuma K, Yamaguchi A. Molecular mechanisms in aging and current strategies to counteract sarcopenia. Curr Aging Sci. 2010;3:90–101.
Pagadala M, Dasarathy S, Eghtesad B, McCullough AJ. Posttransplant metabolic syndrome: an epidemic waiting to happen. Liver Transpl. 2009;15:1662–70.
Friday BB, Mitchell PO, Kegley KM, Pavlath GK. Calcineurin initiates skeletal muscle differentiation by activating MEF2 and MyoD. Differentiation. 2003;71:217–27.
Mullen KD, Denne SC, McCullough AJ, Savin SM, Bruno D, Tavill AS, et al. Leucine metabolism in stable cirrhosis. Hepatology. 1986;6:622–30.
McCullough AJ, Mullen KD, Kalhan SC. Defective nonoxidative leucine degradation and endogenous leucine flux in cirrhosis during an amino acid infusion. Hepatology. 1998;28:1357–64.
Tessari P, Inchiostro S, Barazzoni R, Zanetti M, Orlando R, Biolo G, et al. Fasting and postprandial phenylalanine and leucine kinetics in liver cirrhosis. Am J Physiol. 1994;267:E140–9.
Tessari P, Barazzoni R, Kiwanuka E, Davanzo G, De PG, Orlando R, et al. Impairment of albumin and whole body postprandial protein synthesis in compensated liver cirrhosis. Am J Physiol Endocrinol Metab. 2002;282:E304–11.
Tessari P, Kiwanuka E, Vettore M, Barazzoni R, Zanetti M, Cecchet D, et al. Phenylalanine and tyrosine kinetics in compensated liver cirrhosis: effects of meal ingestion. Am J Physiol Gastrointest Liver Physiol. 2008;295:G598–604.
Morrison WL, Bouchier IA, Gibson JN, Rennie MJ. Skeletal muscle and whole-body protein turnover in cirrhosis. Clin Sci (Lond). 1990;78:613–9.
Ouchi K, Matsubara S, Fukuhara K, Matsuno S. Plasma amino acid abnormalities in liver disease: comparative analysis of idiopathic portal hypertension, extrahepatic portal occlusion and liver cirrhosis. Tohoku J Exp Med. 1989;158:171–8.
Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol. 2003;552:315–24.
Peng S, Plank LD, McCall JL, Gillanders LK, McIlroy K, Gane EJ. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr. 2007;85:1257–66.
Roongpisuthipong C, Sobhonslidsuk A, Nantiruj K, Songchitsomboon S. Nutritional assessment in various stages of liver cirrhosis. Nutrition. 2001;17:761–5.
DiCecco SR, Wieners EJ, Wiesner RH, Southorn PA, Plevak DJ, Krom RA. Assessment of nutritional status of patients with end-stage liver disease undergoing liver transplantation. Mayo Clin Proc. 1989;64:95–102.
Lang CH, Frost RA, Svanberg E, Vary TC. IGF-I/IGFBP-3 ameliorates alterations in protein synthesis, eIF4E availability, and myostatin in alcohol-fed rats. Am J Physiol Endocrinol Metab. 2004;286:E916–26.
Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–9.
Otto A, Collins-Hooper H, Patel K. The origin, molecular regulation and therapeutic potential of myogenic stem cell populations. J Anat. 2009;215:477–97.
Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E1011–8.
Siu PM. Muscle apoptotic response to denervation, disuse, and aging. Med Sci Sports Exerc. 2009;41:1876–86.
Zhang P, Chen X, Fan M. Signaling mechanisms involved in disuse muscle atrophy. Med Hypotheses. 2007;69:310–21.
Filippatos GS, Anker SD, Kremastinos DT. Pathophysiology of peripheral muscle wasting in cardiac cachexia. Curr Opin Clin Nutr Metab Care. 2005;8:249–54.
Freeman LM. The pathophysiology of cardiac cachexia. Curr Opin Support Palliat Care. 2009;3:276–81.
Debigare R, Cote CH, Maltais F. Ubiquitination and proteolysis in limb and respiratory muscles of patients with chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2010;7:84–90.
Kim HC, Mofarrahi M, Hussain SN. Skeletal muscle dysfunction in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3:637–58.
MacIntyre NR. Muscle dysfunction associated with chronic obstructive pulmonary disease. Respir Care. 2006;51:840–7.
Lentine KL, Axelrod D, Abbott KC. Interpreting body composition in kidney transplantation: weighing candidate selection, prognostication, and interventional strategies to optimize health. Clin J Am Soc Nephrol. 2011;6:1238–40.
Sakkas GK, Ball D, Mercer TH, Sargeant AJ, Tolfrey K, Naish PF. Atrophy of non-locomotor muscle in patients with end-stage renal failure. Nephrol Dial Transplant. 2003;18:2074–81.
Streja E, Molnar MZ, Kovesdy CP, Bunnapradist S, Jing J, Nissenson AR, et al. Associations of pretransplant weight and muscle mass with mortality in renal transplant recipients. Clin J Am Soc Nephrol. 2011;6:1463–73.
Donohoe CL, Ryan AM, Reynolds JV. Cancer cachexia: mechanisms and clinical implications. Gastroenterol Res Pract. 2011;2011:601434.
Fearon KC. Cancer cachexia and fat-muscle physiology. N Engl J Med. 2011;365:565–7.
Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. 2012;16:95–131.
Donaghy A, Ross R, Wicks C, Hughes SC, Holly J, Gimson A, et al. Growth hormone therapy in patients with cirrhosis: a pilot study of efficacy and safety. Gastroenterology. 1997;113:1617–22.
Moller S, Becker U, Gronbaek M, Juul A, Winkler K, Skakkebaek NE. Short-term effect of recombinant human growth hormone in patients with alcoholic cirrhosis. J Hepatol. 1994;21:710–7.
Tsien CD, McCullough AJ, Dasarathy S. Late evening snack—exploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol. 2012;27:430–41.
Alvares-da-Silva MR, da ST Reverbel. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition. 2005;21:113–7.
Rantanen T, Harris T, Leveille SG, Visser M, Foley D, Masaki K, et al. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci. 2000;55:M168–73.
Bonefeld K, Moller S. Insulin-like growth factor-I and the liver. Liver Int. 2011;31:911–9.
Conchillo M, de Knegt RJ, Payeras M, Quiroga J, Sangro B, Herrero JI, et al. Insulin-like growth factor I (IGF-I) replacement therapy increases albumin concentration in liver cirrhosis: results of a pilot randomized controlled clinical trial. J Hepatol. 2005;43:630–6.
Sandahl TD, Aagaard NK, Thomsen KL, Grofte T, Greisen J, Christiansen JS, et al. Effects of insulin-like growth factor-I administration on in vivo regulation of urea synthesis in normal subjects and patients with cirrhosis. Liver Int. 2011;31:132–7.
Phillips SM, Tang JE, Moore DR. The role of milk- and soy-based protein in support of muscle protein synthesis and muscle protein accretion in young and elderly persons. J Am Coll Nutr. 2009;28:343–54.
Tang JE, Phillips SM. Maximizing muscle protein anabolism: the role of protein quality. Curr Opin Clin Nutr Metab Care. 2009;12:66–71.
Nicastro H, Artioli GG, Costa AS, Solis MY, da Luz CR, Blachier F, et al. An overview of the therapeutic effects of leucine supplementation on skeletal muscle under atrophic conditions. Amino Acids. 2011;40:287–300.
Drummond MJ, Rasmussen BB. Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis. Curr Opin Clin Nutr Metab Care. 2008;11:222–6.
Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, et al. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab. 2008;294:E392–400.
McGhee A, Henderson JM, Millikan Jr WJ, Bleier JC, Vogel R, Kassouny M, et al. Comparison of the effects of Hepatic-Aid and a Casein modular diet on encephalopathy, plasma amino acids, and nitrogen balance in cirrhotic patients. Ann Surg. 1983;197:288–93.
Horst D, Grace ND, Conn HO, Schiff E, Schenker S, Viteri A, et al. Comparison of dietary protein with an oral, branched chain-enriched amino acid supplement in chronic portal-systemic encephalopathy: a randomized controlled trial. Hepatology. 1984;4:279–87.
Christie ML, Sack DM, Pomposelli J, Horst D. Enriched branched-chain amino acid formula versus a casein-based supplement in the treatment of cirrhosis. JPEN J Parenter Enteral Nutr. 1985;9:671–8.
Swart GR, van den Berg JW, van Vuure JK, Rietveld T, Wattimena DL, Frenkel M. Minimum protein requirements in liver cirrhosis determined by nitrogen balance measurements at three levels of protein intake. Clin Nutr. 1989;8:329–36.
Fiaccadori F, Elia GF, Lehndorff H, Merli M, Pedretti G, Riggio O, et al. The effect of dietary supplementation with branched-chain amino acids vs. casein in patients with chronic recurrent portal systemic encephalopathy: a controlled trial. In: Soeters PB, Wilson JHP, Meijer AJ, Holm E, editors. Advances in ammonia metabolism and hepatic encephalopathy. Amsterdam: Excerpta Medica; 1988. p. 489–97.
Guarnieri GF, Toigo R, Situlin RPG, Faccini L, Marini R, Giuntini D, et al. Muscle biopsy study on malnutrition in patients with liver cirrhosis. In: Capocaccia L, Fischer JE, Rossi-Fanelli F, editors. Hepatic encephalopathy in chronic liver failure. New York: Plenum; 2011.
Egberts EH, Schomerus H, Hamster W, Jurgens P. Branched chain amino acids in the treatment of latent portosystemic encephalopathy. A double-blind placebo-controlled crossover study. Gastroenterology. 1985;88:887–95.
Marchesini G, Dioguardi FS, Bianchi GP, Zoli M, Bellati G, Roffi L, et al. Long-term oral branched-chain amino acid treatment in chronic hepatic encephalopathy. A randomized double-blind casein-controlled trial. The Italian Multicenter Study Group. J Hepatol. 1990;11:92–101.
Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, et al. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle. 2011;1:11.
Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82:1065–73.
Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–7.
Rocchi E, Cassanelli M, Gibertini P, Pietrangelo A, Casalgrandi G, Ventura E. Standard or branched-chain amino acid infusions as short-term nutritional support in liver cirrhosis? JPEN J Parenter Enteral Nutr. 1985;9:447–51.
Teran JC, Mullen KD, McCullough AJ. Glutamine—a conditionally essential amino acid in cirrhosis? Am J Clin Nutr. 1995;62:897–900.
Bonetto A, Penna F, Minero VG, Reffo P, Costamagna D, Bonelli G, et al. Glutamine prevents myostatin hyperexpression and protein hypercatabolism induced in C2C12 myotubes by tumor necrosis factor-alpha. Amino Acids. 2011;40:585–94.
Rennie MJ, Bohe J, Smith K, Wackerhage H, Greenhaff P. Branched-chain amino acids as fuels and anabolic signals in human muscle. J Nutr. 2006;136:264S–8S.
Marchesini G, Marzocchi R, Noia M, Bianchi G. Branched-chain amino acid supplementation in patients with liver diseases. J Nutr. 2005;135:1596S–601S.
Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, et al. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr. 2007;86:373–81.
Burd NA, Tang JE, Moore DR, Phillips SM. Exercise training and protein metabolism: influences of contraction, protein intake, and sex-based differences. J Appl Physiol. 2009;106:1692–701.
Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA, Phillips SM. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol. 2009;587:897–904.
Wilborn CD, Taylor LW, Greenwood M, Kreider RB, Willoughby DS. Effects of different intensities of resistance exercise on regulators of myogenesis. J Strength Cond Res. 2009;23:2179–87.
Lemyze M, Dharancy S, Neviere R, Pruvot FR, Declerck N, Wallaert B. Aerobic capacity in patients with chronic liver disease: very modest effect of liver transplantation. Presse Med. 2010;39:e174–81.
Lemyze M, Dharancy S, Neviere R, Wallaert B. Cardiopulmonary response to exercise in patients with liver cirrhosis and impaired pulmonary gas exchange. Respir Med. 2011;105:1550–6.
Koopman R, Saris WH, Wagenmakers AJ, van Loon LJ. Nutritional interventions to promote post-exercise muscle protein synthesis. Sports Med. 2007;37:895–906.
Koopman R, Verdijk LB, Beelen M, Gorselink M, Kruseman AN, Wagenmakers AJ, et al. Co-ingestion of leucine with protein does not further augment post-exercise muscle protein synthesis rates in elderly men. Br J Nutr. 2008;99:571–80.
Koopman R, van Loon LJ. Aging, exercise, and muscle protein metabolism. J Appl Physiol. 2009;106:2040–8.
Kumar V, Atherton P, Smith K, Rennie MJ. Human muscle protein synthesis and breakdown during and after exercise. J Appl Physiol. 2009;106:2026–39.
Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587:211–7.
Rasmussen BB, Richter EA. The balancing act between the cellular processes of protein synthesis and breakdown: exercise as a model to understand the molecular mechanisms regulating muscle mass. J Appl Physiol. 2009;106:1365–6.
Garcia-Pagan JC, Santos C, Barbera JA, Luca A, Roca J, Rodriguez-Roisin R, et al. Physical exercise increases portal pressure in patients with cirrhosis and portal hypertension. Gastroenterology. 1996;111:1300–6.
Garcia PS, Cabbabe A, Kambadur R, Nicholas G, Csete M. Brief-reports: elevated myostatin levels in patients with liver disease: a potential contributor to skeletal muscle wasting. Anesth Analg. 2010;111:707–9.
von Haehling S, Morley JE, Coats AJ, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle. 2010;1:7-8.
Acknowledgments
The author was supported in part by NIH RO1 DK083414 and NIH UO1 DK 061732. The author of this manuscript certifies that he complies with ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle [146]. Assistance of Ms. Patricia Brandt in extracting and organizing the data from the SRTR registry for Figures 2, 3 and 4 is appreciated.
Conflict of interest
The authors declare that they have no conflict of interest.
The data and analyses reported in the 2009 Annual Data Report of the US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients have been supplied by UNOS and the Minneapolis Medical Research Foundation under contract with HHS/HRSA. The authors alone are responsible for reporting and interpreting these data; the views expressed herein are those of the authors and not necessarily those of the US Government.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Dasarathy, S. Consilience in sarcopenia of cirrhosis. J Cachexia Sarcopenia Muscle 3, 225–237 (2012). https://doi.org/10.1007/s13539-012-0069-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13539-012-0069-3