Abstract
Background
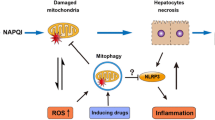
Previous reports indicate that mitochondrial dysfunction is essential for the development of liver injury due to acetaminophen. On the other hand, autophagy, which is a major catabolic pathway, plays a critical role in removing protein aggregates, as well as damaged or excess mitochondria in order to maintain intracellular homeostasis. The aim of this study was to clarify if autophagy is linked to liver injury due to acetaminophen.
Methods
Acetaminophen was administered intraperitoneally to liver-specific Atg7-deficient mice and wild-type mice. A variety of cellular and molecular approaches were used to evaluate the role of autophagy in acetaminophen-induced cell death.
Results
Treatment with acetaminophen induced formation of autophagosomes in hepatocytes from wild-type mice, but not in Atg7-deficient mice. Autophagy deficiency enhanced acetaminophen-induced liver injury and activation of caspase-3 and -7 in the liver. Acetaminophen-induced reactive oxygen species (ROS) production, mitochondrial membrane depolarization, and JNK activation in hepatocytes were accelerated by autophagy deficiency. The combination of cyclosporin A or JNK inhibitor (SP600125) with acetaminophen blunted both acetaminophen-induced apoptotic and necrotic cell death in autophagy-deficient hepatocytes.
Conclusions
Induction of autophagy during acetaminophen treatment plays a pivotal role in the protection against acetaminophen-induced hepatotoxicity through the removal of damaged mitochondria and oxidative stress.






Similar content being viewed by others
References
Fannin R, Russo M, O’Connell T, Gerrish K, Winnike J, Macdonald J, et al. Acetaminophen dosing of humans results in blood transcriptome and metabolome changes consistent with impaired oxidative phosphorylation. Hepatology. 2010;51(1):227–36.
Dargan P, Jones A. Acetaminophen poisoning: an update for the intensivist. Crit Care. 2002;6(2):108–10.
Nagai H, Matsumaru K, Feng G, Kaplowitz N. Reduced glutathione depletion causes necrosis and sensitization to tumor necrosis factor-alpha-induced apoptosis in cultured mouse hepatocytes. Hepatology. 2002;36(1):55–64.
Burcham P, Harman A. Acetaminophen toxicity results in site-specific mitochondrial damage in isolated mouse hepatocytes. J Biol Chem. 1991;266(8):5049–54.
Pumford N, Halmes N. Protein targets of xenobiotic reactive intermediates. Annu Rev Pharmacol Toxicol. 1997;37:91–117.
Masubuchi Y, Suda C, Horie T. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol. 2005;42(1):110–6.
Kim J, He L, Qian T, Lemasters J. Role of the mitochondrial permeability transition in apoptotic and necrotic death after ischemia/reperfusion injury to hepatocytes. Curr Mol Med. 2003;3(6):527–35.
Kim J, Qian T, Lemasters J. Mitochondrial permeability transition in the switch from necrotic to apoptotic cell death in ischemic rat hepatocytes. Gastroenterology. 2003;124(2):494–503.
Klionsky D, Ohsumi Y. Vacuolar import of proteins and organelles from the cytoplasm. Annu Rev Cell Dev Biol. 1999;15:1–32.
Schworer C, Shiffer K, Mortimore G. Quantitative relationship between autophagy and proteolysis during graded amino acid deprivation in perfused rat liver. J Biol Chem. 1981;256(14):7652–8.
Levine B, Klionsky D. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6(4):463–77.
Yoshimori T. Autophagy: a regulated bulk degradation process inside cells. Biochem Biophys Res Commun. 2004;313(2):453–8.
Espert L, Denizot M, Grimaldi M, Robert-Hebmann V, Gay B, Varbanov M, et al. Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. J Clin Invest. 2006;116(8):2161–72.
Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61(2):439–44.
Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, Waguri S, et al. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol. 2008;172(2):454–69.
Cuervo A, Bergamini E, Brunk U, Dröge W, Ffrench M, Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1(3):131–40.
Inami Y, Yamashina S, Izumi K, Ueno T, Tanida I, Ikejima K, Watanabe S. Hepatic steatosis inhibits autophagic proteolysis via impairment of autophagosomal acidification and cathepsin expression. Biochem Biophys Res Commun. 2011;412(4):618–25.
Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169(3):425–34.
Kon K, Kim J, Jaeschke H, Lemasters J. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40(5):1170–9.
Kim J, Nitta T, Mohuczy D, O’Malley K, Moldawer L, Dunn WJ, et al. Impaired autophagy: a mechanism of mitochondrial dysfunction in anoxic rat hepatocytes. Hepatology. 2008;47(5):1725–36.
Leist M, Single B, Castoldi A, Kühnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185(8):1481–6.
Lemasters JV. Necrapoptosis and the mitochondrial permeability transition: shared pathways to necrosis and apoptosis. Am J Physiol. 1999;276(1 Pt 1):G1–6.
Rashed M, Nelson S. Characterization of glutathione conjugates of reactive metabolites of 3′-hydroxyacetanilide, a nonhepatotoxic positional isomer of acetaminophen. Chem Res Toxicol. 1989;2(1):41–5.
Elmore S, Qian T, Grissom S, Lemasters J. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001;15(12):2286–7.
Rodriguez-Enriquez S, Kim I, Currin R, Lemasters J. Tracker dyes to probe mitochondrial autophagy (mitophagy) in rat hepatocytes. Autophagy. 2006;2(1):39–46.
Gunawan B, Liu Z, Han D, Hanawa N, Gaarde W, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131(1):165–78.
Ghosh J, Das J, Manna P, Sil P. Arjunolic acid, a triterpenoid saponin, prevents acetaminophen (APAP)-induced liver and hepatocyte injury via the inhibition of APAP bioactivation and JNK-mediated mitochondrial protection. Free Radic Biol Med. 2010;48(4):535–53.
Conde de la Rosa L, Schoemaker M, Vrenken T, Buist-Homan M, Havinga R, Jansen P, et al. Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: involvement of JNK and ERK MAP kinases. J Hepatol. 2006;44(5):918–29.
Kim B, Ryu S, Song B. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J Biol Chem. 2006;281(30):21256–65.
de Grey A. A proposed refinement of the mitochondrial free radical theory of aging. Bioessays. 1997;19(2):161–6.
Brunk U, Terman A. The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem. 2002;269(8):1996–2002.
Rodriguez-Enriquez S, Kai Y, Maldonado E, Currin R, Lemasters J. Roles of mitophagy and the mitochondrial permeability transition in remodeling of cultured rat hepatocytes. Autophagy. 2009;5(8):1099–106.
Tarantino G, Conca P, Basile V, Gentile A, Capone D, Polichetti G, et al. A prospective study of acute drug-induced liver injury in patients suffering from non-alcoholic fatty liver disease. Hepatol Res. 2007;37(6):410–5.
Acknowledgments
This work was supported in part by Grant-in-Aid (No. 20590797 to SY, No. 21390234 to SW) and High Technology Research Center Grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
535_2011_500_MOESM1_ESM.ppt
Supplementary Fig. 1 Effect of cyclosporine A and rapamycin on APAP-induced liver injury. (a) Mice were killed at 12 h after APAP treatment. Some wild-type and Atg7-KO mice were injected intraperitoneally with cyclosporine A (10 mg/kg) or rapamycin (5 mg/kg) for 3 days prior to APAP treatment. Serum ALT levels of WT and KO mice were measured. Bars represent mean and SEM (n = 4, *; P < 0.05 vs, WT at 12 h after APAP treatment, **; P < 0.05 vs, KO at 12 h after APAP treatment, by ANOVA on ranks). (PPT 144 kb)
Rights and permissions
About this article
Cite this article
Igusa, Y., Yamashina, S., Izumi, K. et al. Loss of autophagy promotes murine acetaminophen hepatotoxicity. J Gastroenterol 47, 433–443 (2012). https://doi.org/10.1007/s00535-011-0500-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-011-0500-0