Abstract
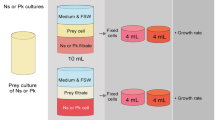
The biological response of increased manganese in seawater was tested experimentally with the oyster species Crassostrea gigas by adding, once per day, a fixed quantity of MnCl2 to the container where the oysters were living. Uptake of Mn2+ in the shell was traced with cathodoluminescence and quantified with a high spatial resolution proton microprobe. The daily addition of MnCl2 resulted in the visualization of distinct growth increments seen simultaneously in both the calcitic shell and the aragonitic ligament. A relation was observed between the addition of Mn2+ to the seawater and incorporation of Mn in the mineral part of the shell. Thus, addition of MnCl2 to seawater is an efficient tool to mark in vivo growth increments in bio-mineralised carbonates.


Similar content being viewed by others
References
Barbin V (1997) Cathodoluminescence of biogenic carbonates. (Key note) 18th regional IAS meeting (Heidelberg, Germany), GAEA heidelbergensis 3:58
Barbin V (2000) Cathodoluminescence of carbonates shells: biochemical vs diagenetic process. In: Pagel M, Barbin V, Blanc Ph, Ohnenstetter D (eds) Cathodoluminescence in Geosciences. Springer, Berlin, pp 303–329
Barbin V, Elfman M, Yang C, Schein E, Roux M, Ramseyer K (1998) Fluctuation des teneurs en manganèse dans les carbonates biogènes: diagenèse ou enregistrement des variations environnementales? Apport de la cathodoluminescence et de la microsonde à protons. Abstract, RST Brest, Soc. Géol. Fr. Edit., Paris. 67. (ISSN 0249 7557)
Barbin V, Ramseyer K, Debenay JP, Schein E, Roux M, Decrouez D (1991) Cathodoluminecence of recent biogenic carbonates: an environmental and ontogenic fingerprint. Geol Mag 128:19–26
Böhn F, Gussone N, Eisenhauer A, Dullo WC, Reynaud S, Paytan A (2006) Calcium isotope fractionation in modern scleractinian corals. Geochim Cosmochim Acta 70:4452–4462
Carriker MR, Palmer RE, Sick LV, Johnson CC (1980) Interaction of mineral elements in seawater and shell oysters (Crassostrea virginica (Gmelin)) cultured in controlled and natural systems. J Exp Mar Bio Ecol 46:279–296
Carriker MR, Swann CP, Prezant RS, Counts CL (1991) Chemical elements in the aragonitic and calcitic microstructural groups of shell of the oyster Crassostrea virginica: a proton probe study. Mar Biol 109:287–297
Cravo A, Bebianno MJ, Foster P (2004) Partitioning of trace metals between soft tissues and shells of Patella aspera. Environ Int 30:87–98
de Riclès A, Livage J (2004) An introduction to biomineralization: diversity and unity. CR Palevol 3:435–441
Dubois PH, Chen CP (1989) Calcification in echinoderms. In: Jangoux M, Lawrence JM (eds) Echinoderm studies, vol 3. Balkema, Rotterdam, pp 109–172
El Ali A, Barbin V, Calas G, Cervelle B, Ramseyer K, Bouroulec J (1993) Mn2+-activated luminescence in dolomite, calcite and magnesite: quantitative determination of manganese and site distribution by EPR and CL spectroscopy. Chem Geol 104:189–202
Elfman M, Kristiansson P, Malmqvist KG, Pallon J, Sjöland KA, Utui RJ, Yang C (1997) New CAMAC based data acquisition and beam control system for Lund nuclear microprobe. Nucl Instrum Methods B 130:123–126
Elfman M, Kristiansson P, Malmqvist KG, Pallon J (1999) The layout and performance of the LUND nuclear microprobe trigger and data acquisition system. Nucl Instrum Methods B 158:141–145
Fred C, Andrus T, Crowe DE (2000) Geochemical analysis of Crassostrea virginica as a method to determine season capture. J Arch Sci 27:33–42
Götze J (2002) Potential of cathodoluminescence (CL) microscopy and spectroscopy for tne analysis of minerals and materials. Anal Bioanal Chem 374:703–708
Johansson SAE, Cambell JL (1988) PIXE: a novel technique for elemental analysis. Wiley, New York, 347 pp
Lowenstam HT (1981) Minerals formed by organisms. Science 211:1126
Lowenstam HT, Weiner S (1989) On biomineralization. Oxford University Press, Oxford, 324 pp
Malmqvist KG, Hylten G, Hult M, Håkansson K, Knox JM, Larsson NPO, Nilsson C, Pallon J, Schofield R, Swietlicki E, Tapper UAS, Yang C (1993) Dedicated accelerator and microprobe line. Nucl Instrum Methods B 77:3–7
Marshall DJ (1988) Cathodoluminescence of geological materials. Unwin Hyman, Boston, 146 pp
Nordstrom DK, Plummer LN, Wigley TML, Ball JW, Jenne EA, Bassett RL, Crerar DA, Florence TM, Fritz B, Hoffman M, Holdren GR Jr, Lafon GM, Mattigod SV, McDuff RE, Morel F, Reddy MM, Sposito G, Thrailkill J (1979) Comparison of computerized chemical models for equilibrium calculations in aqueous systems. In: Jenne EA (ed) Chemical modelling in aqueous systems, ACS symposium series 93. American Chemical Society, Washington, 892 pp
Ramseyer K, Fischer J, Matter A, Eberhardt P, Geiss J (1989) A cathodoluminescence microscope for low intensity luminescence. J Sediment Petrol 59:619–622
Rousseau M, Plouguerné E, Wan G, Lopez E, Fouchereau-Peron M (2003) Biomineralization markers during a phase of active growth in Pinctada margaritifera. Comp Biochem Physiol A 135:271–278
Rosenberg GD (1980) An ontogenetic approach to the environmental significance of bivalve shell chemistry. In: Rhoads DC, Lutz RA (eds) Skeletal growth of aquatic organisms. Plenum, New York, pp 33–168
Stenzel HB (1962) Aragonite in the resilium of oysters. Science 136:1121–1122
Szefer P, Frelek K, Szefer K, Lee ChB, Kim BS, Warzocha J (2002) Distribution and relationships of trace metals in soft tissue, byssus and shells of Mytilus edulis trossulus from the southern Baltic. Environ Pollut 120:423–444
Waldichuk M (1974) Some biological concerns in heavy metal pollution In: Vernberg FJ, Vernberg WB (eds) Pollution and physiology of marine organisms. Academic, New York, pp 1–55
Wilbur KM, Saleuddin ASM (1983) Shell formation. In: Saleuddin ASM, Wilbur KM (eds) The Mollusca, vol 4. Academic, New York, pp 235–287
Witkowski FW, Blundell DJ, Gutteridge P, Horbury AD, Oxtoby NH, Quing H (2000) Video cathodoluminescence microscopy of diagenetic cements and its applications. Mar Petrol Geol 17:1085–1093
Acknowledgments
The authors would like to thank Jens Götze and Anton Eisenhauer for their very helpful comments. We also thank M. Elfman, S. Hocquet, C. Piquet, M. Roux, E. Schein, E. Yang, and IFREMER for their help at different moments of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barbin, V., Ramseyer, K. & Elfman, M. Biological record of added manganese in seawater: a new efficient tool to mark in vivo growth lines in the oyster species Crassostrea gigas . Int J Earth Sci (Geol Rundsch) 97, 193–199 (2008). https://doi.org/10.1007/s00531-006-0160-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00531-006-0160-0