Abstract
Many dinoflagellate species are bioluminescent, which is one of the anti-predation mechanisms in these species. In addition, dinoflagellate species experience a wide range of salinities in the ocean. However, the effects of salinity on their bioluminescence intensity has only been investigated for one species. Here, we explored the effect of salinity on the bioluminescence intensity of the heterotrophic dinoflagellate Noctiluca scintillans NSDJ2010 feeding on the chlorophyte Dunaliella salina, the heterotrophic dinoflagellate Polykrikos kofoidii PKJH1607 feeding on the dinoflagellate Alexadrium minutum, and the autotrophic dinoflagellate Alexandrium mediterraneum AMYS1807. Moreover, to determine the cell volume and growth effects on bioluminescence intensity, the cell volume and growth rate of three bioluminescent dinoflagellates were simultaneously investigated. The mean 200-s-integrated bioluminescence intensity (BL) per cell, equivalent to the total bioluminescence, of N. scintillans, P. kofoidii, and A. mediterraneum was significantly affected by salinity and increased with increasing salinity from 10 to 40. The results of the present study suggest that the total bioluminescence of N. scintillans, P. kofoidii, and A. mediterraneum in offshore and oceanic waters is greater than that in estuarine waters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bioluminescence is the production of visible light by living organisms (Wilson and Hastings 2013), and various bioluminescent organisms exist in the oceans (Shimomura 2006; Widder 2010; Wilson and Hastings 2013; Cusick and Widder 2020). Dinoflagellates are among the most common bioluminescent organisms found in the sea (Haddock et al. 2010; Cusick and Widder 2020). Bioluminescence in dinoflagellates is a defense mechanism against predators, that startle their predators or makes a predator to be seen by higher predators in darkness (Burkenroad 1943; Esaias and Curl 1972; Haddock et al. 2010; Valiadi and Iglesias-Rodriguez 2013; Prevett et al. 2019). Furthermore, the bioluminescence of dinoflagellates can lower the ingestion rate of predators (Esaias and Curl 1972; White 1979; Prevett et al. 2019). Therefore, dinoflagellate bioluminescence can affect the function of marine planktonic food webs.
Light emission from dinoflagellates is stimulated by physical stressors, such as waves, ships, submarines, fish, mammals, and zooplankton predation (Esaias and Curl 1972; Morin 1983; Rohr et al. 1998; Latz et al. 2004, 2008; Maldonado and Latz 2007; Cusick and Widder 2014). Many bioluminescent dinoflagellates form red tides and harmful algal blooms (Anderson et al. 2012; Cusick and Widder 2020; Jeong et al. 2021). Dinoflagellate bioluminescence is sometimes observed as a night-time optical phenomenon during a dinoflagellate bloom (Seliger et al. 1970; Seliger 1989; Wilson and Hastings 2013; Martínez et al. 2016; Rodríguez 2020; Domingues 2021; Detoni et al. 2023). Consequently, the movements of ships and submarines can be detected from the air at night, which affects military operations (Lynch 1978, 1981; Lapota 2003, 2005; Moline et al. 2005; Haddock et al. 2010).
Total bioluminescence intensity (TBI) of dinoflagellates is known to be affected by several biological, physical, and chemical factors. For example, prey species and its density (Buskey et al. 1992, 1994; Buskey 1995; Latz and Jeong 1996), water temperature (Sweeney 1981; Jess 1985; Park et al. 2024), and light intensity in daytime affect TBI (Buskey et al. 1992; Li et al. 1996). Dinoflagellates usually experience a wide salinity range, which may affect TBI. However, prior to the present study, the effects of salinity on TBI of only one dinoflagellate, Pyrocystis lunula, were explored (Craig et al. 2003).
Among the dinoflagellate species, the heterotrophic dinoflagellate Noctiluca scintillans is known to produce the highest bioluminescence intensity, except for Pyrocystis noctiluca (Sweeney 1971; Buskey et al. 1992; Cusick and Widder 2014). This dinoflagellate is able to feed on diverse prey species, such as bacteria, phytoplankton, mixoplankton, and eggs of metazoans (Buskey 1995; Kirchner et al. 1996; Quevedo et al. 1999; Drits et al. 2013; Zhang et al. 2016; Stauffer et al. 2017) and often blooms in the global ocean (Anantharaman et al. 2010; Harrison et al. 2011; Lotliker et al. 2018; Valiadi et al. 2019; Jeong et al. 2021). Red tide patches of N. scintillans sometimes extend hundreds of kilometers in coastal and oceanic waters (Mohanty et al. 2007; Kopuz et al. 2014; Qi et al. 2019). The patches appear as long red or pink patches due to the digestion of prey during the daytime, but they emit a glowing blue light at night (Huang and Qi 1997; Padmakumar et al. 2010; Temnykh et al. 2022). Another heterotrophic dinoflagellate Polykrikos kofoidii has a bioluminescent capability and is able to feed on diverse dinoflagellate species (Buskey et al. 1992; Matsuyama et al. 1999; Matsuoka et al. 2000; Jeong et al. 2001, 2003, 2010; Kang et al. 2018, 2023; Kim et al. 2019). This dinoflagellate is commonly observed in coastal waters of many countries, particularly in Australia, Denmark, Kingdom of the Netherlands, Japan, Norway, United Kingdom, United States, Republic of Korea, Republic of South Africa, and Tanzania (Buskey et al. 1992; Matsuoka et al. 2000; Gómez 2003; Ocean Survey 20/202013; Tillmann and Hoppenrath 2013; Lim et al. 2017; Prabowo and Agusti 2019; OBIS 2023) Recently, the bioluminescence capability of the autotrophic dinoflagellate Alexandrium mediterraneum has been reported (Park et al. 2021); this species is found in the coastal waters of Greece, Italy, Spain, and Korea (John et al. 2003; Lilly et al. 2007; Penna et al. 2008; Aguilera-Belmonte et al. 2011; Giulietti 2017; Park et al. 2021). These three dinoflagellates have been found in a wide range of salinities (Elbrächter and Qi 1998; Matsuoka et al. 2000; Mohamed and Mesaad 2007; Harrison et al. 2011; Chuenniyom et al. 2012; Lim et al. 2017; OBIS 2023). Therefore, to understand bioluminescence in seas, it is necessary to explore whether the bioluminescence intensity of these dinoflagellates is affected by salinity.
In the present study, the bioluminescence intensities of N. scintillans, P. kofoidii, and A. mediterraneum were determined as a function of salinity. Moreover, the relationships between their bioluminescence intensity and cell volume and growth rate were investigated. The effects of cell volume of these dinoflagellates were investigated at same salinity, and the equations of linear regression between cell volume and bioluminescence intensity were determined. These results provide a basis for understanding salinity effects on the bioluminescence of heterotrophic and autotrophic dinoflagellates, as well as dinoflagellate bioluminescence in the sea.
Materials and methods
Preparation of experimental organisms
Cells of Noctiluca scintillans NSDJ2010, Polykrikos kofoidii PKJH1607, and Alexandrium mediterraneum AMYS1807 were isolated from water samples collected from Dangjin, Jangheung, and Yeosu, Korea, and a clonal culture of each dinoflagellate species was established (Table 1). The cultures of N. scintillans and P. kofoidii were fed with the chlorophyte Dunaliella salina and dinoflagellate Alexandrium minutum as prey, respectively. A dense culture of N. scintillans (approximately 5–10 cells mL−1) was transferred to a 250-mL flask (Falcon; Corning Inc., NewYork, USA) that contained D. salina (approximately 10,000 cells mL−1). In addition, a dense culture of P. kofoidii (approximately 50–70 cells mL−1) was transferred to a 250-mL flask containing A. minutum (approximately 2,000 cells mL−1). The N. scintillans and P. kofoidii cultures were incubated under 20 μmol photons m−2 s−1 of light-emitting diode (LED; FS-075MU, 6500 K; Suram Inc., Suwon, Korea) and 14: 10 h light: dark cycle. The A. mediterraneum culture was grown in F/2 seawater medium without silicate (hereafter F/2; Guillard and Ryther 1962) and was incubated under 100 μmol photons m−2 s−1 of LED and 14: 10 h light: dark cycle. The cultures of all three bioluminescent dinoflagellates and their prey species were incubated at 20℃ and a salinity of 30.
Experimental setup
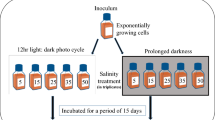
Experiments 1–3 were designed to investigate the effects of salinity on the bioluminescence intensities of N. scintillans, P. kofoidii, and A. mediterraneum. For each target salinity experiment, only filtered seawater or culture media containing the target salinity was used. The target salinity of seawater or culture medium was established by diluting natural seawater with deionized water or by adding sea salt to natural seawater, which has a salinity of 30. Salinity was measured using a YSI MultiLab 4010-3W (YSI, Yellow Springs, OH, United States). The flasks containing each bioluminescent species and their prey species were incubated under constant conditions or gradually transitioned to the target salinity for nine days to avoid any shock that may occur when a large change in the incubation condition occurs rapidly (Fig. S1). All of the cultures in experiments 1–3 were conducted in a culture chamber at 20 ℃. During the pre-incubation period, a 5-mL aliquot was pipetted from each flask at 2-day intervals and fixed with Lugol’s solution and then the abundance of each species was determined. In all cultures, the mobility and morphology of cells were examined under a light microscope every other day to determine the amount of cell death. If almost bioluminescent dinoflagellate cells in the flask were dead by increasing or decreasing salinity, the experiments under the target salinity were not conducted.
For experiment 1, dense N. scintillans cultures were transferred to eight 250-mL flasks containing D. salina and filtered seawater. A dense culture of D. salina was transferred to eight flasks containing F/2 medium. During the pre-incubation period, cells of N. scintillans at a salinity of 30 were subsequently transferred to flasks at final salinities of 25, 20, 15, 10, and 5 by dilution. In contrast, cells of N. scintillans at a salinity of 30 were transferred to flasks with final salinities of 35 and 40 by the addition of salts. Almost all cells of N. scintillans in the flask did not survive at a salinity of 5; therefore, the experiments were conducted at salinities of 10, 15, 20, 25, 30, 35, and 40. The bioluminescence intensity and growth rate of N. scintillans at each target salinity were measured using the method described by Park et al. (2024). After pre-incubation, three 1-mL aliquots were taken from each flask, and the density of each experimental species was determined using a light microscope (Olympus BX51; Olympus Co., Tokyo, Japan) at a magnification of 40–200 × . When prey cells in the N. scintillans culture were undetectable, the initial concentrations of N. scintillans and D. salina were achieved by adding a predetermined volume of culture having a known cell density to each well of the experimental well plate (Table 2). Triplicate experimental wells (mixtures of N. scintillans and D. salina), triplicate prey control wells (D. salina only), and triplicate predator control wells (N. scintillans only) were set up in 6-well culture plates (Fig. 1). One milliliter of the F/2 medium at each target salinity was added to each well for each target salinity. A culture of N. scintillans with each target salinity was filtered with a 0.2-µm disposable syringe filter (DISMIC-25CS type, 25 mm; Advantec, Toyo Roshi Kaisha Ltd., Chiba, Japan), and then the filtrates were added to the prey control well at the same volume as that of the predator culture added to the experiment well. A culture of D. salina with each target salinity was filtered with a 0.2-µm disposable syringe filter, and then the filtrates were added to the predator control well at the same volume as that of the prey culture added to the experiment well. The final volume of each well was 10 mL. These plates were incubated for two days at 20 μmol photons m−2 s−1 produced by LED lights with a cycle of 14: 10 h light: dark cycle. At the beginning and end of the experiments, 4-mL aliquots were removed from each well and fixed with Lugol’s solution with a final concentration of 5%, respectively, and the densities were determined. After a 2-day incubation, the bioluminescence intensity, growth rate, and cell volume of N. scintillans were determined as described in the “Measurement of bioluminescence intensity” and “Measurements of the growth rate and cell volume” sections.
For experiment 2, dense P. kofoidii cultures were transferred to eight 250-mL flasks containing A. minutum and filtered seawater. Alexandrium minutum cells were transferred to eight flasks containing L1 medium (L1 without silicate; Guillard and Hargraves 1993). After screening the salinity by pre-incubation, the target salinities of 10, 15, 20, 25, 30, 35, and 40 were established. The procedures for establishment, incubation, and enumeration in this experiment were the same as those in Experiment 1, except for the target and prey species and culture medium (L1 without silicate instead of F/2).
For experiment 3, eight flasks were prepared and filled with fresh F/2 medium and A. mediterraneum cells. After screening the salinity by pre-incubation, target salinities of 19, 23, 25, 30, 35, 37, and 40 were established. By adding a predetermined volume of culture to 38-mL flask and filled with F/2 medium that adjust to each target salinity (final volume = 30 mL), the initial concentrations of A. mediterraneum were achieved. Culture flasks were established in triplicate at each target salinity. These flasks were incubated for four days at 100 μmol photons m−2 s−1 produced by LED lights with a cycle of 14:10 h light: dark cycle. Four-mL aliquots were removed from each flask at the beginning and end of the experiments and fixed with Lugol’s solution. After a 4-day incubation, the bioluminescence intensity, growth rate, and cell volume of A. mediterraneum were determined.
Measurement of bioluminescence intensity
The bioluminescence intensity in each experiment was measured using the methods described by Park et al. (2021, 2024). In experiments 1 and 2, after 2-day incubation, a 200-μL aliquot of filtered seawater in each 6-well experimental plate was pipetted into a 96-well white plate (Corning Life Sciences, Amsterdam, Netherlands). Next, three cells of N. scintillans (or P. kofoidii) in each well with the target salinity were transferred to the wells of the 96-well white plate. Five replicate wells containing 3 N. scintillans (or P. kofoidii) cells and five replicate wells containing only freshly filtered seawater were established. The 96-well white plates were placed in the darkness for 3 h at 20 ℃ (Biggley et al. 1969; Krasnow et al. 1980; Lindström et al. 2017). Bioluminescence of the target dinoflagellate was stimulated chemically by adding 50 μL of 1 M acetic acid to the well of the 96-well white plate (Hastings and Sweeney 1957; Fogel and Hastings 1972; Sweeney 1986). Using a GloMax Navigator microplate luminometer (Promega, Madison, WI, USA), stimulated bioluminescence was measured for 200 s. In the experiment 3, a 200-μL aliquot of a culture of A. mediterraneum in each flask that was incubated for 4 days at the target salinity was placed in a well of a 96-well white plate. Five replicate wells in 96-well white plates were used. The bioluminescence intensity was measured as described above.
The bioluminescence intensity per cell per second in each well of a 96-well white plate was determined by subtracting the mean value of the bioluminescence intensity per second in the five control wells (without dinoflagellate cells) from the bioluminescence intensity value per second in each experimental well (with dinoflagellate cells) and then dividing the value by the number of cells in the experimental well. The bioluminescence intensity per cell per second for 200 s in each well was determined and the integrated bioluminescence intensity per cell for 200 s (BL per cell) was calculated. The value of BL per cell is almost equivalent to the total bioluminescence intensity per cell or the total luminescence capacity per cell (von Dassow and Latz 2002) because almost all dinoflagellate cells did not emit bioluminescence after 200 s. The mean bioluminescence intensity per cell for 200 s [mean BL per cell; relative light unit (RLU) cell−1] was calculated by averaging BL per cell in five wells.
Measurements of the growth rate and cell volume
After homogenizing the cells in each well to determine the cell densities at the beginning and end of the experiments, 4-mL aliquots were taken from each well or flask and fixed with Lugol’s solution at a final concentration of 5%. All or > 200 cells of a bioluminescent dinoflagellate and prey were enumerated in a Sedgewick-Rafter counting chamber (Jeong et al. 2001).
The specific growth rate of each target dinoflagellate, μ (d−1), was calculated as follows:
where C0 and Ct are the concentrations of each target dinoflagellate at 0 and 48 h for N. scintillans and P. kofoidii or at 0 and 96 h for A. mediterraneum, respectively.
The cell length and width of N. scintillans, P. kofoidii, and A. mediterraneum preserved in 5% Lugol’s solution at the end of each experiment were measured by 20 cells on a confocal dish with cover glasses using a digital camera (Zeiss AxioCam 506; Carl Zeiss Ltd., Göttingen, Germany). The cell volumes of the three bioluminescent dinoflagellates were estimated to be spherical for N. scintillans and A. mediterraneum and cylindrical for P. kofoidii.
Statistical analyses
To examine the effect of salinity on the bioluminescence intensity of each target bioluminescent dinoflagellate, a one-way analysis of variance (ANOVA) with post-hoc Tukey’s honest significant difference (HSD) test was performed (Tukey 1949). Normality was tested using Shapiro–Wilk’s W, and homogeneity of variance was tested using Levene’s tests. If the data were not normally distributed, they were analyzed using the Kruskal–Wallis test and the Mann–Whitney U test with Bonferroni correction (P < 0.05; Mann and Whitney 1947; Kruskal and Wallis 1952). Correlations among bioluminescence intensity, salinity, growth rate, and cell volume in each experiment were examined using the Pearson correlation coefficient or non-parametric Spearman’s rank correlation coefficient. Before applying the statistical tests, using the Shapiro–Wilk W test, the normality of the data was confirmed. Using the SPSS ver. 25.0 (IBM-SPSS Inc., Armonk, NY, USA), statistical analyses were performed.
Results
Cell shape, cell volume, and growth rates of the dinoflagellates at different salinities
Salinities of 5–40 affected the cell shape of N. scintillans NSDJ2010, P. kofoidii PKJH1607, and A. mediterraneum AMYS1807 (Fig. 2). Cells of N. scintillans and P. kofoidii burst at a salinity of 5, whereas A. mediterraneum cells burst at a salinity of 16. Cells of N. scintillans and P. kofoidii at a salinity of 10 swelled. However, at other salinities, N. scintillans, P. kofoidii, and A. mediterraneum maintained their normal cell shapes.
The range of cell volumes of N. scintillans was 4.56–9.05 × 107 µm3 (Fig. 3a). The cell volume of N. scintillans was largest at a salinity of 10 and rapidly decreased at a salinity of 15. However, there was no further decrease in the cell volume from a salinity of 15 to 40. The statistical analysis revealed no significant correlation between the cell volume of N. scintillans and salinity (Spearman’s rank correlation coefficient, rs = − 0.10, P = 0.24). The maximum cell volume of P. kofoidii was 3.46 × 105 µm3 at a salinity of 10, and it gradually decreased to 1.53 × 105 µm3 at a salinity of 35 (Fig. 3b). The cell volume of P. kofoidii was significantly correlated with salinity (Spearman’s rank correlation coefficient, rs = − 0.62, P < 0.01). The range of cell volumes of A. mediterraneum was 2.11–2.70 × 104 µm3, reaching a maximum at a salinity of 19 (Fig. 3c). The cell volume of A. mediterraneum significantly decreased as the salinity increased (Spearman’s rank correlation coefficient, rs = − 0.20, P = 0.02).
Cell volume of Noctiluca scintillans feeding on Dunaliella salina (a) and Polykrikos kofoidii feeding on Alexandrium minutum (b) and Alexandrium mediterraneum (c) as a function of the salinity range. Specific growth rates of N. scintillans feeding on D. salina (d) and P. kofoidii feeding on A. minutum (e) and A. mediterraneum (f) as a function of the salinity range. Symbols represent treatment means ± standard error
The specific growth rates of N. scintillans feeding on D. salina at salinities of 10 and 40 were almost zero (− 0.01 d−1 and 0.08 d−1, respectively), whereas those at the other salinities were 0.39–0.57 d−1, with the maximum growth rate at a salinity of 30 (Fig. 3d). The specific growth rate of P. kofoidii feeding on A. minutum at a salinity of 10 was almost zero (− 0.04 d−1), whereas the growth rates at other salinities were 0.19–0.68 d−1, with the maximum growth rate at a salinity of 20 (Fig. 3e). The specific growth rate of A. mediterraneum at a salinity of 19 was almost zero (− 0.05 d−1), whereas the growth rates at the other salinities were 0.13–0.34 d−1, with the maximum growth rate at a salinity of 30 (Fig. 3f). The ranges of growth rates of D. salina and A. minutum at salinities of 10 − 40 were 0.04–0.35 d−1 and − 0.02–0.26 d−1, respectively (Fig. S2).
Correlations between the growth rate and cell volume of the dinoflagellates
The growth rates of N. scintillans, P. kofoidii, and A. mediterraneum were not significantly correlated with their cell volumes (Fig. 4; Pearson’s correlation; r = − 0.71, P = 0.07 for N. scintillans; r = − 0.30, P = 0.51 for P. kofoidii; r = − 0.53, P = 0.23 for A. mediterraneum). The cell volumes of the three dinoflagellates reached a maximum when their growth rates were negative.
Cell volume of Noctiluca scintillans feeding on Dunaliella salina (a) and Polykrikos kofoidii feeding on Alexandrium minutum (b) and Alexandrium mediterraneum (c) as a function of the growth rate of N. scintillans, P. kofoidii, and A. mediterraneum, respectively. Symbols represent treatment means ± standard error
Effects of salinity on the bioluminescent intensity of Noctiluca scintillans
The mean BL per cell values of N. scintillans feeding on D. salina were significantly affected by salinity (Kruskal–Wallis test; H6 = 23.56, P < 0.01). The mean BL per cell of N. scintillans ranged from 0.45 × 107 to 2.78 × 107 RLU cell−1, reaching a maximum at a salinity of 40 (Fig. 5). The values were divided into two subsets (Mann–Whitney U test with Bonferroni correction, P < 0.05). Significant positive correlations were seen between mean BL per cell of N. scintillans and salinity (Pearson’s correlation; r = 0.77, P < 0.001).
Bar graph of mean 200-s-integrated bioluminescence intensity (BL) per cell of Noctiluca scintillans feeding on Dunaliella salina as a function of salinity at 10–40. Mann–Whitney U test with Bonferroni correction after the Kruskal–Wallis test resulted in significantly different subsets for the mean BL per cell (a; P < 0.05). Different letters on the top of the bars indicate a significant difference between the subsets. Bars represent treatment mean values ± standard error (n = 5)
Effects of salinity on the bioluminescent intensity of Polykrikos kofoidii
The mean BL per cell values of P. kofoidii feeding on A. minutum were significantly affected by salinity (Kruskal–Wallis test, H6 = 25.1, P < 0.001). The mean BL per cell of P. kofoidii at salinity 10–40 ranged from 0.31 × 105 to 3.40 × 105 RLU cell−1, reaching a maximum at a salinity of 40 (Fig. 6). Bonferroni correction (P < 0.05) revealed that mean BL per cell values of P. kofoidii were divided into three subsets. Significant positive correlations were seen between mean BL per cell of P. kofoidii and salinity (Pearson’s correlation: r = 0.86, P < 0.001).
Bar graph of mean 200-s-integrated bioluminescence intensity (BL) per cell of Polykrikos kofoidii feeding on Alexandrium minutum as a function of salinity at 10–40. Mann–Whitney U test with Bonferroni correction after the Kruskal–Wallis test resulted in significantly different subsets for the mean BL per cell (a; P < 0.05). Different letters on the top of the bars indicate a significant difference among the subsets. Bars represent treatment mean values ± standard error (n = 5)
Effects of salinity on the bioluminescent intensity of Alexandrium mediterraneum
The mean BL per cell values of A. mediterraneum were significantly affected by salinity (Fig. 7; Kruskal–Wallis test, H6 = 22.42, P < 0.01). The mean BL per cell of A. mediterraneum at 19–40 ranged from 0.83 × 104 to 4.49 × 104 RLU cell−1, reaching a maximum at a salinity of 40. The values were divided into two subsets (Mann–Whitney U test with Bonferroni correction, P < 0.05). Significant positive correlations were seen between mean BL per cell of A. mediterraneum and salinity (Spearman’s rank correlation; rs = 0.64, P < 0.001).
Bar graphs of mean 200-s-integrated bioluminescence intensity (BL) per cell of Alexandrium mediterraneum as a function of salinity at 19–40. Mann–Whitney U test with Bonferroni correction after the Kruskal–Wallis test resulted in significantly different subsets for the mean BL per cell (a; P < 0.05). Different letters on the top of the bars indicate a significant difference between the subsets. Bars represent treatment mean values ± standard error (n = 5)
Correlations between the growth rate and bioluminescence intensity of the dinoflagellates
The mean BL per cell was not significantly correlated with the growth of N. scintillans, P. kofoidii, or A. mediterraneum (Fig. 8; Pearson’s correlation; r = − 0.34, P = 0.46 for N. scintillans; r = − 0.11, P = 0.81 for P. kofoidii; r = − 0.13, P = 0.79 for A. mediterraneum). However, the highest mean BL per cell of N. scintillans, P. kofoidii, and A. mediterraneum was achieved at a salinity of 40, at which the positive growth rates were lowest. The lowest mean BL per cell of N. scintillans, P. kofoidii, and A. mediterraneum was achieved at the lowest salinity tested, at which the growth rates were negative.
The mean 200-s-integrated bioluminescence intensity (BL) per cell of Noctiluca scintillans feeding on Dunaliella salina (a) and Polykrikos kofoidii feeding on Alexandrium minutum (b) and Alexandrium mediterraneum (c) as a function of the growth rate of N. scintillans, P. kofoidii, and A. mediterraneum, respectively
Correlations between the cell volume and bioluminescence intensity of three dinoflagellates
When the BL per cell values of N. scintillans, P. kofoidii, and A. mediterraneum were pooled at a salinity of 25, log (BL per cell) showed a significant positive correlation with log (cell volume) (Fig. 9; Spearman’s rank correlation; rs = 0.95, P < 0.01). Similar patterns were observed at salinities of 30, 35, and 40 (Spearman’s rank correlation; rs = 0.95, P < 0.01 for salinity 30; rs = 0.95, P < 0.01 for salinity 35; rs = 0.95, P < 0.01 for salinity 40).
The logarithm of 200-s-integrated bioluminescence intensity (BL) per cell as a function of the logarithm of the cell volume of Noctiluca scintillans (red circle), Polykrikos kofoidii (blue circle), and Alexandrium mediterraneum (green circle) at a salinity of 25 (a), 30 (b), 35 (c), and 40 (d). The regression line, indicated by the black line, represented the corresponding equation and the shaded areas represent the 95% confidence interval. The equations of linear regression: a log (BL per cell) = 0.74 log (cell volume) + 1.06, r2 = 0.97 (P < 0.01); b log (BL per cell) = 0.81 log (cell volume) + 0.79, r2 = 0.96 (P < 0.01); c log (BL per cell) = 0.83 log (cell volume) + 0.83, r2 = 0.97 (P < 0.01); d log (BL per cell) = 0.80 log (cell volume) + 1.23, r2 = 0.98 (P < 0.01). Symbols represent single treatment
Discussion
To the best of our knowledge, this is the first study to explore the effects of salinity on the bioluminescence intensity of the heterotrophic dinoflagellates N. scintillans NSDJ2010, P. kofoidii PKJH1607, and an autotrophic dinoflagellate A. mediterraneum AMYS1807. The results of the present study extended the number of dinoflagellates investigated for the effects of salinity on bioluminescence intensity from one to four.
Although the growth rates of N. scintillans were similar at salinities of 10 and 40, that is, almost zero, the cell volume of N. scintillans was 1.7 times larger at a salinity of 10 than at a salinity of 40. Due to osmosis, the lowest salinity is likely to cause the cells to become swollen, while the highest salinity is likely to cause the cells to shrink. Furthermore, the growth rates of P. kofoidii were the lowest and second lowest at salinities of 10 and 40, respectively, but the cell volume of P. kofoidii was 2.2 times larger at a salinity of 10 than at a salinity of 40. N. scintillans and P. kofoidii do not have theca on their cell surface (Soyer 1970; Kwok et al. 2023). Thus, osmosis may largely change the volume of naked N. scintillans and P. kofoidii cells. In estuarine waters, which have a salinity of 10, large cells of N. scintillans and P. kofoidii can be found, and in salty waters with a salinity of 40, small cells of N. scintillans and P. kofoidii can be found. However, their abundance is likely to be low under both conditions due to their low growth rate.
The mean BL per cell values of N. scintillans, P. kofoidii, and A. mediterraneum were lowest at the lowest salinity tested, even though the cell volumes were largest. Considering their zero or negative growth rates, the cells found at these lowest salinities were likely to be almost dead and their bioluminescence became dim. On the contrary, the mean BL per cell values of N. scintillans, P. kofoidii, and A. mediterraneum were highest at the highest salinity, even though their cell volumes were small and their growth rates were low.
Craig et al. (2003) also showed that the bioluminescence intensity of Pyrocystis lunula was the lowest at a salinity of 17 and highest at a salinity of 38 when the tested salinity range was 17–48 (Table 3). The bioluminescence intensities of P. lunula at salinities of 43 and 48 were slightly lower than those at salinities of 38. Therefore, the results of Craig et al. (2003) and our experiments clearly show that the bioluminescence intensity of dinoflagellates is high at high salinity and low at low salinity if the cells survive.
At salinities higher or lower than the isosmotic point, the intracellular ion concentration is altered by osmoregulation or osmoadaptation (Wegmann 1986; Kirst 1990; Mayfield and Gates 2007; Suescún-Bolívar and Thomé 2015). Increasing extracellular salinity increases cytosolic Ca2+ and K+ in various organisms (Dickson and Kirst 1987a, b; Lynch and Läuchli 1988; Lynch et al. 1989; Lee and Liu 1999; Laohavisit et al. 2013; Seifikalhor et al. 2019); it also increases the accumulation of intracellular K+ inside phytoplankton cells (e.g. Dickson and Kirst 1987a, b). The concentrations of Na+, K+, Ca2+, and Mg2+ in the cytosol of N. scintillans can be altered by water efflux and influx into cells (Nawata and Sibaoka 1976). During bioluminescence in dinoflagellates, Ca2+ is released from intracellular Ca stores or absorbed from extracellular sources, which increases the Ca2+ concentration inside the cytosol and triggers bioluminescence (Haley et al. 1995; Lumpkin and Hudspeth 1995; von Dassow and Latz 2002). Von Dassow and Latz (2002) reported that the bioluminescence of Lingulodinium polyedra was stimulated by an increase in extracellular Ca2+ and K+. Thus, increased salinity may lead to increased ion concentrations in the extracellular environment (Dittmar 1884; Forchhammer 1865; Trask 1936), which can enhance the influx of Ca2+ and K+ into the cytosol and ultimately increase bioluminescence. In the present study, deionized water and sea salt were used to achieve the target salinities. Therefore, there are potential discrepancies associated with the means of dilution and the concentration of seawater in natural marine environments.
The range of salinities in global oceans, except for extreme cases of high salinity, is 0.5 − 42 (Brewer and Dyrssen 1985; John et al. 1990; Samuelsson 1996; Matthäus and Schinke 1999; Talley 2002; Emeis et al. 2003; Meier and Kauker 2003; Feistel et al. 2010; Urquhart et al. 2012). The range of salinities at which cells have been observed is 10 − 39 for N. scintillans, 8 − 38 for P. kofoidii, and 17 − 37 for A. mediterraneum (Buskey et al. 1992; Elbrächter and Qi 1998; Gómez 2003; John et al. 2003; Mohamed and Mesaad 2007; Lilly et al. 2007; Penna et al. 2008; Aguilera-Belmonte et al. 2011; Harrison et al. 2011; Chuenniyom et al. 2012; Ocean Survey 20/20 2013; Tillmann and Hoppenrath 2013; Yoo et al. 2013; Giulietti 2017; Lim et al. 2017; Prabowo and Agusti 2019; Eom et al. 2021; Park et al. 2021; Ok et al. 2023). The range of BL per cell values of N. scintillans in global oceans is expected to be 0.45 × 107 to 2.78 × 107 RLU cell−1, a sixfold difference between cells at the upper and lower ends of the range. The range of BL per cell values of P. kofoidii in global oceans is expected to be 0.31 × 105 to 3.40 × 105 RLU cell−1, which is an 11-fold difference. The range of BL per cell values of A. mediterraneum in global oceans is expected to be 0.83 × 104 to 3.34 × 104 RLU cell−1, which is a fourfold difference. Thus, when considering the effects of salinity, the difference in the BL per cell values in global oceans is likely to be larger among P. kofoidii cells than among N. scintillans or A. mediterraneum cells.
The bright bioluminescence of dinoflagellates is known to startle predatory copepods or draw the attention of higher-order predators to the copepod’s location (White 1979; Esaias and Curl 1972; Prevett et al. 2019). Various genera of dinoflagellates, except for N. scintillans and P. kofoidii, show mechanisms for inhibiting the stimulation of bioluminescence in the presence of light (Hamman et al. 1981a, b; Sweeney et al. 1983; Sullivan and Swift 1994; Buskey et al. 1992; Li et al. 1996). Consequently, grazing deterrence by A. mediterraneum using its luminescence occurs primarily at night, while N. scintillans and P. kofoidii can be effective both at night and when ambient light is dim. The mean BL per cell values of N. scintillans, P. kofoidii, and A. mediterraneum are greater at high salinities, which are usually found in offshore and oceanic waters, than at low salinities, which are usually found in estuarine waters. Therefore, in offshore and oceanic waters, the bright bioluminescence of dinoflagellates will likely reduce predation by copepods. However, in estuarine waters, the dim bioluminescence of dinoflagellates may not reduce predation by copepods. Furthermore, high turbidity in estuarine waters may mask the bioluminescence of dinoflagellates, which may not affect predation by copepods.
During World War I, a German U-boat was discovered and sunk due to bioluminescence in the Strait of Gibraltar (Fernandes and Bajaj 2022). Bioluminescence produced by marine organisms has been studied by some countries to detect warships and submarines at night (Lynch 1978, 1981; Lapota 2003, 2005; Moline et al. 2005). Warships operate in most marine countries and submarines operate in several countries, such as the United States, the United Kingdom, South Korea, China, and Russia (Global Firepower 2023). Warships and submarines travel from their bases to oceans worldwide to carry out operations, and they encounter a diverse range of salinity levels (Pritchard 1952; Lundeberg 1963; Charette and Buesseler 2004; Feistel et al. 2010). During the daytime, the movement of submarines is tracked using information obtained by military reconnaissance satellites (Chriqui 2006; Shabbir et al. 2019). However, it is difficult to acquire this information at night and thus, bioluminescence stimulated by operating submarines can be used for detection (Moline et al. 2005). The Strait of Gibraltar connects the Atlantic Ocean and the Mediterranean Sea, and has a salinity range of 35 to 38 (Bryden et al. 1994; Flecha et al. 2019). Blooms of N. scintillans and P. kofoidii have been reported in the Atlantic Ocean and the Mediterranean Sea (Harrison et al. 2011; Provoost and Enevoldsen 2023). Based on the results of the present study, blooms of N. scintillans and P. kofoidii would produce close to their brightest bioluminescence at these high salinities and the waves generated by moving warships and submarines are likely to stimulate these dinoflagellates to produce bright bioluminescence, which can be easily detected.
The U.S. Norfolk Naval Base, currently the world’s largest naval facility, is located in Chesapeake Bay (Conger 2019). Inflow from the Elizabeth River affects the salinity in this area, which is less than 16 (Pritchard 1952; Charette and Buesseler 2004; Urquhart et al. 2012). Furthermore, the area exhibits consistently high turbidity throughout the year, which is attributed to the inflow of suspended particulate matter (Shuyler et al. 1995). Considering the scattered and reduced bioluminescence due to turbidity and salinity, detecting bioluminescence stimulated by submarines or ships within the base may be challenging.
In the present study, N. scintillans showed almost zero growth at the lowest salinity tested, potentially resulting in similar mean BL per cell values for the lowest salinity and salinities of 15–25. Meanwhile, negative growth rates were observed for P. kofoidii and A. mediterraneum at the lowest salinity, indicating sublethal salinity stress. Under this condition, P. kofoidii, and A. mediterraneum showed the lowest mean BL per cell. A reduction in the bioluminescence of dinoflagellates is also observed when they are exposed to various sublethal stressors, such as heavy metals, ammonia, polycyclic aromatic hydrocarbons, and sediment-bound metals and organic compounds (Hannan et al. 1986; Lapota et al. 1993, 1995, 1997, 2007; Lapota 1998; Okamoto et al. 1999; Rosen et al. 2008; Hildenbrand et al. 2015; Perin et al. 2022). Consequently, the findings of our study suggest that salinity can serve as an indicator of sublethal stress, leading to a reduction in dinoflagellate bioluminescence.
The rates of inhibition of dinoflagellate bioluminescence, used to assess acute and sublethal toxic effects, have been investigated under exposure to a toxicant for durations ranging from 2 h to 11 days (Hannan et al. 1986; Lapota et al. 1993; Craig et al 2003; Rosen et al. 2008; Stauber et al. 2008; Hildenbrand et al. 2015; Perin et al. 2022). In the present study, the duration of acclimation to the target salinity ranged from 9 to 14 days, and Craig et al. (2003) had a duration of 4 h. In these investigations, the bioluminescence of dinoflagellates was significantly affected by salinity. Changes in salinity significantly had a notable impact on the inhibition of bioluminescence in P. lunula induced by sodium dodecyl sulfate (Craig et al. 2003). Therefore, salinity should be carefully considered when performing bioassays, as it can affect the rate of inhibition of bioluminescence. The findings of the present study provide a basis for understanding the impact of environmental factors on dinoflagellate bioluminescence and may further contribute to our understanding of the function of dinoflagellate bioluminescence in the marine planktonic food web and application of bioluminescence.
Data availability
Enquiries about data availability should be directed to the authors.
References
Aguilera-Belmonte A, Inostroza I, Franco JM, Riobó P, Gómez PI (2011) The growth, toxicity and genetic characterization of seven strains of Alexandrium catenella (Whedon and kofoid) balech 1985 (Dinophyceae) isolated during the 2009 summer outbreak in southern chile. Harmful Algae 12:105–112. https://doi.org/10.1016/j.hal.2011.09.006
Anantharaman P, Thirumaran G, Arumugam R, Kannan RRR, Hemalatha A, Kannathasan A, Sampathkumar P, Balasubramanian T (2010) Monitoring of Noctiluca bloom in Mandapam and Keelakarai coastal waters; southeast coast of India. Recent Res Sci Technol 2:51–58
Anderson DM, Alpermann TJ, Cembella AD, Collos Y, Masseret E, Montresor M (2012) The globally distributed genus Alexandrium: multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 14:10–35. https://doi.org/10.1016/j.hal.2011.10.012
Biggley WH, Swift E, Buchanan RJ, Seliger HH (1969) Stimulable and spontaneous bioluminescence in the marine dinoflagellates, Pyrodinium bahumensis, Gonyaulax polyedra, and Pyrocystis lunula. J Gen Physiol 54:96–122. https://doi.org/10.1085/jgp.54.1.96
Brewer PG, Dyrssen D (1985) Chemical oceanography of the Persian gulf. Prog Oceanogr 14:41–55. https://doi.org/10.1016/0079-6611(85)90004-7
Bryden HL, Candela J, Kinder TH (1994) Exchange through the Strait of Gibraltar. Prog Oceanogr 33:201–248. https://doi.org/10.1016/0079-6611(94)90028-0
Burkenroad MD (1943) A possible function of bioluminescence. J Mar Res 5:161–164
Buskey EJ (1995) Growth and bioluminescence of Noctiluca scintillans on varying algal diets. J Plankton Res 17:29–40. https://doi.org/10.1093/plankt/17.1.29
Buskey EJ, Strom S, Coulter C (1992) Biolumiscence of heterotrophic dinoflagellates from texas coastal waters. J Exp Mar Biol Ecol 159:37–49. https://doi.org/10.1016/0022-0981(92)90256-a
Buskey EJ, Coulter CJ, Brown SL (1994) Feeding, growth and bioluminescence of the heterotrophic dinoflagellate Protoperidinium huberi. Mar Biol 121:373–380. https://doi.org/10.1007/bf00346747
Charette MA, Buesseler KO (2004) Submarine groundwater discharge of nutrients and copper to an urban subestuary of Chesapeake Bay (Elizabeth river). Limnol Oceanogr 49:376–385. https://doi.org/10.4319/lo.2004.49.2.0376
Chriqui J (2006) Military applications of India’s space program: the military surveillance and reconnaissance system. Institute of peace and conflict studies. http://www.ipcs.org/comm_select.php?articleNo=2043. Accessed 20 June 2006
Chuenniyom W, Meksumpun C, Meksumpun S (2012) Impacts of nutrients and related environmental factors on distribution and size structure of Noctiluca scintillans populations of the Eutrophic Tha Chin Estuary, Thailand. Water Sci Technol 65:1994–2002. https://doi.org/10.2166/wst.2012.099
Conger J (2019) An overview of the DOD installations enterprise Heritage foundation https://www.heritage.org/military-strength-topical-essays/2019-essays/overview-the-dod-installations-enterprise Accessed 20 November 2020
Craig JM, Klerks PL, Heimann K, Waits JL (2003) Effects of salinity, pH and temperature on the re-establishment of bioluminescence and copper or SDS toxicity in the marine dinoflagellate Pyrocystis lunula using bioluminescence as an endpoint. Environ Pollut 125:67–275. https://doi.org/10.1016/s0269-7491(03)00059-9
Cusick KD, Widder EA (2014) Intensity differences in bioluminescent dinoflagellates impact foraging efficiency in a nocturnal predator. Bull Mar Sci 90:797–811. https://doi.org/10.5343/bms.2013.1059
Cusick KD, Widder EA (2020) Bioluminescence and toxicity as driving factors in harmful algal blooms: ecological functions and genetic variability. Harmful Algae 98:101850. https://doi.org/10.1016/j.hal.2020.101850
Detoni AMS, Navarro G, Garrido JL, Rodríguez F, Hernández-Urcera J, Caballero I (2023) Mapping dinoflagellate blooms (Noctiluca and Alexandrium) in aquaculture production areas in the NW Iberian Peninsula with the Sentinel-2/3 satellites. Sci Total Environ 868:161579. https://doi.org/10.1016/j.scitotenv.2023.161579
Dickson DMJ, Kirst GO (1987a) Osmotic adjustment in marine eukaryotic algae: the role of inorganic ions, quaternary ammonium, tertiary sulphonium and carbohydrate solutes: I. Diatoms and a Rhodophyte New Phytol 106:645–655. https://doi.org/10.1111/j.1469-8137.1987.tb00165.x
Dickson DMJ, Kirst GO (1987b) Osmotic adjustment in marine eukaryotic algae: the role of inorganic ions, quaternary ammonium, tertiary sulphonium and carbohydrate solutes: II. Prasinophytes and Haptophytes New Phytol 106:645–655. https://doi.org/10.1111/j.1469-8137.1987.tb00166.x
Dittmar W (1884) Report on the scientific results of the exploring voyage of HMS challenger. Phys Chem 1:1–251
Domingues RB (2021) News media coverage and public reactions to a red tide at the Algarve Coast (Southern portugal). J Spat Organ Dyn 9:176–188
Drits AV, Nikishina AB, Sergeeva VM, Solov’ev KA (2013) Feeding, respiration, and excretion of the Black Sea Noctiluca scintillans MacCartney in summer. Oceanology 53:442–450. https://doi.org/10.1134/s0001437013040036
Elbrächter M, Qi Z (1998) Aspects of Noctiluca (dinophyceae) population dynamics. In: Anderson DM (ed) Physiological ecology of harmful algal blooms. Springer, Berlin, pp 315–335
Emeis KC, Struck U, Blanz T, Kohly A, Voβ M (2003) Salinity changes in the central Baltic Sea (NW Europe) over the last 10000 years. The Holocene 13:411–421. https://doi.org/10.1191/0959683603hl634
Eom SH, Jeong HJ, Ok JH, Park SA, Kang HC, You JH, Lee SY, Yoo YD, Lim AS, Lee MJ (2021) Interactions between common heterotrophic protists and the dinoflagellate Tripos furca: implication on the long duration of its red tides in the South Sea of Korea in 2020. Algae 36:25–36. https://doi.org/10.4490/algae.2021.36.2.22
Esaias WE, Curl HC Jr (1972) Effect of dinoflagellate bioluminescence on copepod ingestion rates. Limnol Oceanogr 17:901–906. https://doi.org/10.4319/lo.1972.17.6.0901
Feistel R, Weinreben S, Wolf H, Seitz S, Spitzer P, Adel B, Nausch G, Schneider B, Wright DG (2010) Density and absolute salinity of the Baltic Sea 2006–2009. Ocean Sci 6:3–24. https://doi.org/10.5194/os-6-3-2010
Fernandes MS, Bajaj P (2022) Implications for India of Noctiluca scintillans. In: Chauhan VAP, Lahiri CD, Bajaj DP (eds) Maritime perspectives 2022: transitioning from a brown to a blue economy in the face of climate change. National Maritime Foundation, New Delhi, pp 186–202
Flecha S, Pérez FF, Murata A, Makaoui A, Huertas IE (2019) Decadal acidification in Atlantic and Mediterranean water masses exchanging at the Strait of Gibraltar. Sci Rep 9:15533. https://doi.org/10.1038/s41598-019-52084-x
Fogel M, Hastings JW (1972) Bioluminescence: mechanism and mode of control of scintillon activity. Proc Natl Acad Sci 69:690–693. https://doi.org/10.1073/pnas.69.3.690
Forchhammer G (1865) IV. On the composition of sea-water in the different parts of the ocean. Philos Trans R Soc London 155:203–262. https://doi.org/10.1098/rstl.1865.0004
Giulietti S (2017) Identification of several strains of the potentially toxic genus Alexandrium from the Bilbao estuary. Dissertation, University of Bologna
Global Firepower (2023) 2023 submarine fleet strength by country. Available at: https://www.globalfirepower.com/navy-submarines.php
Gómez F (2003) Checklist of Mediterranean free-living dinoflagellates. Bot Mar 46:215–242. https://doi.org/10.1515/BOT.2003.021
Guillard RRL, Hargraves PE (1993) Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia 32:234–236
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can J Microbiol 8:229–239. https://doi.org/10.1139/m62-029
Haddock SHD, Moline MA, Case JF (2010) Bioluminescence in the sea. Annu Rev Mar Sci 2:443–493. https://doi.org/10.1146/annurev-marine-120308-08aaaaaaaaaaaa1028
Haley A, Russell AJ, Wood N, Allan AC, Knight M, Campbell AK, Trewavas AJ (1995) Effects of mechanical signaling on plant cell cytosolic calcium. Proc Natl Acad Sci USA 92:4124–4128. https://doi.org/10.1073/pnas.92.10.4124
Hamman JP, Biggley WH, Seliger HH (1981a) Action spectrum for the photoinhibition of bioluminescence in the marine dinoflagellate Dissodinium lunula. Photochem Photobiol 33:741–747. https://doi.org/10.1111/j.1751-1097.1981.tb05483.x
Hamman JP, Biggley WH, Seliger HH (1981b) Photoinhibition of stimulable bioluminescence in marine dinoflagellates. Photochem Photobiol 33:909–914. https://doi.org/10.1111/j.1751-1097.1981.tb05511.x
Hannan PJ, Stiffey AV, Jarvis BB (1986) Bioluminescence as the Basis for the Detection of Tricothecenes. Naval Research Laboratory, Washington, p 25
Harrison PJ, Furuya K, Glibert PM, Xu J, Liu HB, Yin K, Lee JHW, Anderson DM, Ho AYT (2011) Geographical distribution of red and green Noctiluca scintillans. Chin J Oceanol Limnol 29:807–831. https://doi.org/10.1007/s00343-011-0510-z
Hastings JW, Sweeney BM (1957) The luminescent reaction in extracts of the marine dinoflagellate, Gonyaulax polyedra. J Cell Comp Physiol 49:209–225. https://doi.org/10.1002/jcp.1030490205
Hildenbrand ZL, Osorio A, Carlton DD, Fontenot BE, Walton JL, Hunt LR, Oka H, Hopkins D, Bjorndal B, Schug KA (2015) Rapid analysis of eukaryotic bioluminescence to assess potential groundwater contamination events. J Chem. https://doi.org/10.1155/2015/957608
Huang C, Qi Y (1997) The abundance cycle and influence factors on red tide phenomena of Noctiluca scintillans (Dinophyceae) in Dapeng Bay, the South China Sea. J Plank Res 19:303–318. https://doi.org/10.1093/plankt/19.3.303
Jeong HJ, Kim SK, Kim JS, Kim ST, Yoo YD, Yoon JY (2001) Growth and grazing rates of the heterotrophic dinoflagellate Polykrikos kofoidii on red-tide and toxic dinoflagellates. J Eukar Microbiol 48:298–308. https://doi.org/10.1111/j.1550-7408.2001.tb00318.x
Jeong HJ, Park KH, Kim JS, Kang HJ, Kim CH, Choi HJ, Kim YS, Park JY, Park MG (2003) Reduction in the toxicity caused by a toxic dinoflagellate Gymnodinium catenatum by the feeding of the heterotrophic dinoflagellate Polykrikos kofoidii. Aquat Microb Ecol 31:307–312. https://doi.org/10.3354/ame031307
Jeong HJ, Yoo YD, Kim JS, Seong KA, Kang NS, Kim TH (2010) Growth, feeding, and ecological roles of the mixotrophic and heterotrophic dinoflagellates in marine planktonic food webs. Ocean Sci J 45:65–91. https://doi.org/10.1007/s12601-010-0007-2
Jeong HJ, Kang HC, Lim AS, Jang SH, Lee K, Lee SY, Ok JH, You JH, Kim JH, Lee KH, Park SA, Eom SH, Yoo YD, Kim KY (2021) Feeding diverse prey as an excellent strategy of mixotrophic dinoflagellates for global dominance. Sci Adv 7:eabe4214. https://doi.org/10.1126/sciadv.abe4214
Jess MM (1985) The comparative effect of temperature on mechanically stimulated flash responses of three bioluminescent dinoflagellates, Pyrocystis fusiformis, Pyrocystis noctiluca and Noctiluca miliaris. Dissertation, University of California, Santa Barbara
John VC, Coles SL, Abozed AI (1990) Seasonal cycles of temperature, salinity and water masses of the western Arabian Gulf. Oceanol Acta 13:273–281
John U, Fensome RA, Medlin LK (2003) The application of a molecular clock based on molecular sequences and the fossil record to explain biogeographic distributions within the Alexandrium tamarense “species complex” (Dinophyceae). Mol Biol Evol 20:1015–1027. https://doi.org/10.1093/molbev/msg105
Kang HC, Jeong HJ, Kim SJ, You JH, Ok JH (2018) Differential feeding by common heterotrophic protists on 12 different Alexandrium species. Harmful Algae 78:106–117. https://doi.org/10.1016/j.hal.2018.08.005
Kang HC, Jeong HJ, Lim AS, Ok JH, You JH, Park SA, Eom SH (2023) Feeding by common heterotrophic protists on the mixotrophic dinoflagellate Ansanella granifera (Suessiaceae). Algae 37:57–70. https://doi.org/10.4490/algae.2023.38.2.24
Kim SJ, Jeong HJ, Kang HC, You JH, Ok JH (2019) Differential feeding by common heterotrophic protists on four Scrippsiella species of similar size. J Phycol 55:868–881. https://doi.org/10.1111/jpy.12864
Kirchner M, Sahling G, Uhlig G, Gunkel W, Klings KW (1996) Does the red tide-forming dinoflagellate Noctiluca scintillans feed on bacteria? Sarsia 81:45–55. https://doi.org/10.1080/00364827.1996.10413610
Kirst GO (1990) Salinity tolerance of eukaryotic marine algae. Annu Rev Plant Biol 41:21–53. https://doi.org/10.1146/annurev.pp.41.060190.000321
Kopuz U, Feyzioglu AM, Valente A (2014) An unusual red-tide event of Noctiluca scintillans (Macartney) in the Southeastern Black Sea. Turk J Fish Aquat Sci 14:261–268. https://doi.org/10.4194/1303-2712-v14_1_28
Krasnow R, Dunlap JC, Taylor W, Hastings JW, Vetterling W, Gooch V (1980) Circadian spontaneous bioluminescent glow and flashing of Gonyaulax polyedra. J Comp Phvsiol 138:19–26. https://doi.org/10.1007/bf00688730
Kruskal WH, Wallis WA (1952) Use of ranks in one-criterion variance analysis. J Am Stat Assoc 47:583–621. https://doi.org/10.1080/01621459.1952.10483441
Kwok ACM, Chan WS, Wong JTY (2023) Dinoflagellate amphiesmal dynamics: cell wall deposition with ecdysis and cellular growth. Mar Drugs 21:70. https://doi.org/10.3390/md21020070
Laohavisit A, Richards SL, Shabala L, Chen C, Colaço RD, Swarbreck SM, Shaw E, Dark A, Shabala S, Shang Z, Davies JM (2013) Salinity-induced calcium signaling and root adaptation in arabidopsis require the calcium regulatory protein annexin. Plant Physiol 163:253–262. https://doi.org/10.1104/pp.113.217810
Lapota D (1998) Long-term and seasonal changes in dinoflagellate bioluminescence in the Southern California Bight. University of California, Santa Barbara
Lapota D (2003) Buoy-mounted bioluminescence sensor (BioBuoy) for special operations 3. Intelligence, Surveillance and Reconnaissance, SSC San Diego Biennial Review 2003, SSC San Diego, San Diego, pp 142–145
Lapota D (2005) Night time surveillance of harbors and coastal areas using bioluminescence camera and buoy systems. Proc SPIE Int Soc Opt Eng 5780:128–137. https://doi.org/10.1117/12.609624
Lapota D, Moskowitz GJ, Rosenberger DE, Grovhoug JG (1993) The use of stimulable bioluminescence from marine dinoflagellates as a means of detecting toxicity in the marine environment. In: Gorsuch JW (ed) Environmental toxicology and risk assessment. American Society for Testing and Materials, Philadelphia, pp 3–18. https://doi.org/10.1520/STP1179-EB
Lapota D, Liu CH, Rosenberger DE (1997) Use of a rapid biolumineseent bioassay (QuikLite) using dinoflagellates to assess potential toxicity on pore waters from sediment at Navy sites. Society of Environmental Toxicity and Chemistry, 18th Annual Meeting, 16–20 November, 1997, San Francisco, CA
Lapota D, Osorio AR, Liao C, Bjorndal B (2007) The use of bioluminescent dinoflagellates as an environmental risk assessment tool. Mar Pollut Bull 54:1857–1867. https://doi.org/10.1016/j.marpolbul.2007.08.008
Latz MI, Jeong HJ (1996) Effect of red tide dinoflagellate diet and cannibalism on the bioluminescence of the heterotrophic dinoflagellates Protoperidinium spp. Mar Ecol Prog Ser 132:275–285. https://doi.org/10.3354/meps132275
Latz MI, Juhl AR, Ahmed AM, Elghobashi SE, Rohr J (2004) Hydrodynamic stimulation of dinoflagellate bioluminescence: a computational and experimental study. J Exp Biol 207:1941–1951. https://doi.org/10.1242/jeb.00973
Latz MI, Bovard M, VanDelinder V, Segre E, Rohr J, Groisman A (2008) Bioluminescent response of individual dinoflagellate cells to hydrodynamic stress measured with millisecond resolution in a microfluidic device. J Exp Biol 211:2865–2875. https://doi.org/10.1242/jeb.011890
Lee TM, Liu CH (1999) Correlation of decreased calcium contents with proline accumulation in the marine green macroalga Ulva fasciata exposed to elevated NaCl contents in seawater. J Exp Bot 50:1855–1862. https://doi.org/10.1093/jxb/50.341.1855
Li Y, Swift E, Buskey EJ (1996) Photoinhibition of mechanically stimulable bioluminescence in the heterotrophic dinoflagellate Protoperidinium depressum (Pyrrophyta). J Phycol 32:974–982. https://doi.org/10.1111/j.0022-3646.1996.00974.x
Lilly EL, Halanych KM, Anderson DM (2007) Species boundaries and global biogeography of the Alexandrium tamarense complex (Dinophyceae). J Phycol 43:1329–1338. https://doi.org/10.1111/j.1529-8817.2007.00420.x
Lim AS, Jeong HJ, Seong KA, Lee MJ, Kang NS, Jang SH, Lee KH, Park JY, Jang TY, Yoo YD (2017) Ichthyotoxic Cochlodinium polykrikoides red tides in South Sea, Korea in 2014: II. Heterotrophic protists and their grazing impacts on red-tide organisms. Algae 32:199–222. https://doi.org/10.4490/algae.2017.32.8.25
Lindström J, Grebner W, Rigby K, Selander E (2017) Effects of predator lipids on dinoflagellate defence mechanisms-increased bioluminescence capacity. Sci Rep 7:1–9. https://doi.org/10.1038/s41598-017-13293-4
Lotliker AA, Baliarsingh SK, Trainer VL, Wells ML, Wilson C, Bhaskar TU, Samanta A, Shahimol SR (2018) Characterization of oceanic Noctiluca blooms not associated with hypoxia in the Northeastern Arabian Sea. Harmful Algae 74:46–57. https://doi.org/10.1016/j.hal.2018.03.008
Lumpkin EA, Hudspeth AJ (1995) Detection of Ca2+ entry through mechanosensitive channels localizes the site of mechanoelectrical transduction in hair cells. Proc Natl Acad Sci USA 92:10297–10301. https://doi.org/10.1073/pnas.92.22.10297
Lundeberg PK (1963) The German Naval Critique of the U-Boat Campaign, 1915–1918. Mil Aff 27:105–118. https://doi.org/10.2307/1984204
Lynch RV (1978) The occurrence and distribution of surface bioluminescence in the oceans during 1966 through 1977. U.S. Naval Research Laboratory, Washington, USA
Lynch RV (1981) Analysis of fleet reports of bioluminescence in the Indian Ocean. U.S. Naval Research Laboratory, Washington, USA
Lynch J, Läuchli A (1988) Salinity affects intracellular calcium in corn root protoplasts. Plant Physiol 87:351–356. https://doi.org/10.1104/pp.87.2.351
Lynch J, Polito VS, Läuchli A (1989) Salinity stress increases cytoplasmic ca activity in maize root protoplasts. Plant Physiol 90:1271–1274. https://doi.org/10.1104/pp.90.4.1271
Maldonado EM, Latz MI (2007) Shear-stress dependence of dinoflagellate bioluminescence. Biol Bull 212:242–249. https://doi.org/10.2307/25066606
Mann HB, Whitney DR (1947) On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat 18:50–60. https://doi.org/10.1214/aoms/1177730491
Martínez A, Méndez S, Fabre A (2016) First record of bioluminescence of Alexandrium fraterculus (dinoflagellate), in the Uruguayan coast, South Western Atlantic Ocean. Pan-Am J Aquat Sci 11:356–360
Matsuoka K, Cho HJ, Jacobson DM (2000) Observations of the feeding behavior and growth rates of the heterotrophic dinoflagellate Polykrikos kofoidii (Polykrikaceae, Dinophyceae). Phycologia 39:82–86. https://doi.org/10.2216/i0031-8884-39-1-82.1
Matsuyama Y, Miyamoto M, Kotani Y (1999) Grazing impacts of the heterotrophic dinoflagellate Polykrikos kofoidii on a bloom of Gymnodinium catenatum. Aquat Microb Ecol 17:91–98. https://doi.org/10.3354/ame017091
Matthäus W, Schinke H (1999) The influence of river runoff on deep water conditions of the Baltic Sea. In: Blomqvist (ed) Biological, Physical and Geochemical Features of Enclosed and Semi-enclosed Marine Systems: Proceedings of the Joint BMB 15 and ECSA 27 Symposium, 9–13 June 1997. Springer Netherlands, Åland Islands, pp 1–10
Mayfield AB, Gates RD (2007) Osmoregulation in anthozoan–dinoflagellate symbiosis. Comp Biochem Physiol Part a: Mol Integr Physiol 147:1–10. https://doi.org/10.1016/j.cbpa.2006.12.042
Meier HM, Kauker F (2003) Sensitivity of the Baltic Sea salinity to the freshwater supply. Clim Res 24:231–242. https://doi.org/10.3354/cr024231
Mohamed ZA, Mesaad I (2007) First report on Noctiluca scintillans blooms in the red sea off the coasts of Saudi Arabia: consequences of eutrophication. Oceanologia 49:337–351
Mohanty AK, Satpathy KK, Sahu G, Sasmal SK, Sahu BK, Panigrahy RC (2007) Red tide of Noctiluca scintillans and its impact on the coastal water quality of the near-shore waters, off the Rushikulya River, Bay of Bengal. Current Sci 93:616–618
Moline M, Bissett P, Blackwell S, Mueller J, Sevadjian J, Trees C, Zaneveld R (2005) An autonomous vehicle approach for quantifying bioluminescence in ports and harbors. Proc SPIE 5780:81–87
Morin JG (1983) Coastal bioluminescence: patterns and functions. Bull Mar Sci 33:787–817
Nawata T, Sibaoka T (1976) Ionic composition and pH of the vacuolar sap in marine dinoflagellate Noctiluca. Plant Cell Physiol 17:265–272. https://doi.org/10.1093/oxfordjournals.pcp.a075278
OBIS 2023 Ocean Biodiversity Information System Intergovernmental Oceanographic Commission of UNESCO www.obis.org
Ocean Survey 20/20 2013 International polar year and census of antarctic marine life ross sea voyage (TAN0802) biodiversity data Southwestern Pacific OBIS, National Institute of Water and Atmospheric Research, Wellington, New Zealand, 8748 records http://nzobisipt.elasticbeanstalk.com/resource.do?
Ok JH, Jeong HJ, Kang HC, You JH, Park SA, EomSH KJK, Yoo YD (2023) Protists in hypoxic waters of Jinhae Bay and Masan Bay, Korea, based on metabarcoding analyses: emphasizing surviving dinoflagellates. Algae 38:265–281. https://doi.org/10.4490/algae.2023.38.12.6
Okamoto OK, Shao L, Hastings JW, Colepicolo P (1999) Acute and chronic effects of toxic metals on viability, encystment and bioluminescence in the dinoflagellate Gonyaulax polyedra. Comp Biochem Physiol Part C 123:75–83. https://doi.org/10.1016/S0742-8413(99)00013-4
Padmakumar KB, SreeRenjima G, Fanimol CL, Menon NR, Sanjeevan VN (2010) Preponderance of heterotrophic Noctiluca scintillans during a multi-species diatom bloom along the southwest coast of India. Int J Oceanogr 4:55–63
Park SA, Jeong HJ, Ok JH, Kang HC, You JH, Eom SH, Yoo YD, Lee MJ (2021) Bioluminescence capability and intensity in the dinoflagellate Alexandrium species. Algae 36:299–314. https://doi.org/10.4490/algae.2021.36.12.6
Park SA, Jeong HJ, Ok JH, Kang HC, You JH, Eom SH, Yoo YD, Lee MJ (2024) Estimation of bioluminescence intensity of the dinoflagellates Noctiluca scintillans, Polykrikos kofoidii, and Alexandrium mediterraneum populations in Korean waters using cell abundance and water temperature. Algae 39:1–16. https://doi.org/10.4490/algae.2024.39.3.10
Penna A, Fraga S, Masó M, Giacobbe MG, Bravo I, Garcés E, Vila M, Bertozzini E, Andreoni F, Luglié A, Vernesi C (2008) Phylogenetic relationships among the Mediterranean Alexandrium (Dinophyceae) species based on sequences of 5.8 S gene and Internal Transcript Spacers of the rRNA operon. Eur J Phycol 43:163–178. https://doi.org/10.1080/09670260701783730
Perin LS, Moraes GV, Galeazzo GA, Oliveira AG (2022) Bioluminescent dinoflagellates as a bioassay for toxicity assessment. Int J Mol Sci 23:13012. https://doi.org/10.3390/ijms232113012
Prabowo DA, Agusti S (2019) Free-living dinoflagellates of the central Red Sea, Saudi Arabia: variability, new records and potentially harmful species. Mar Pollut Bull 141:629–648. https://doi.org/10.1016/j.marpolbul.2019.03.012
Prevett A, Lindström J, Xu J, Karlson B, Selander E (2019) Grazer-induced bioluminescence gives dinoflagellates a competitive edge. Curr Biol 29:R564–R565. https://doi.org/10.1016/j.cub.2019.05.019
Pritchard DW (1952) Salinity distribution and circulation in the Chesapeake Bay estuarine system. J Mar Res 11:106–123 (https://elischolar.library.yale.edu/journal_of_marine_research/763)
P Provoost H Enevoldsen 2023 Harmful algal event database (HAEDAT). Version 3.18. IOC harmful algal bloom programme Sampling event dataset https://doi.org/10.25607/0wdrmq accessed via GBIF.org on 2023–12–16.
Qi L, Tsai SF, Chen Y, Le C, Hu C (2019) In search of red Noctiluca scintillans blooms in the East China Sea. Geophys Res Lett 46:5997–6004. https://doi.org/10.1029/2019gl082667
Quevedo M, Gonzalez-Quiros R, Anadon R (1999) Evidence of heavy predation by Noctiluca scintillans on Acartia clausi (Copepoda) eggs off the central Cantabrian coast (NW Spain). Oceanol Acta 22:127–131. https://doi.org/10.1080/00364827.1996.10413610
Rodríguez F (2020) Las Noctilucas son para el verano. https://fitopasion.com/2020/08/las-noctilucas-son-para-el-verano.html
Rohr J, Latz MI, Fallon S, Nauen JC, Hendricks E (1998) Experimental approaches towards interpreting dolphin-stimulated bioluminescence. J Exp Biol 201:1447–1460. https://doi.org/10.1242/jeb.201.9.1447
Rosen G, Osorio-Robayo A, Rivera-Duarte I, Lapota D (2008) Comparison of bioluminescent dinoflagellate (QwikLite) and bacterial (Microtox) rapid bioassays for the detection of metal and ammonia toxicity. Arch Environ Contam Toxicol 54:606–611. https://doi.org/10.1007/s00244-007-9068-3
Samuelsson M (1996) Interannual salinity variations in the Baltic Sea during the period 1954–1990. Cont Shelf Res 16:1463–1477. https://doi.org/10.1016/0278-4343(95)00082-8
Seifikalhor M, Aliniaeifard S, Shomali A, Azad N, Hassani B, Lastochkina O, Li T (2019) Calcium signaling and salt tolerance are diversely entwined in plants. Plant Signal Behav 14:1665455. https://doi.org/10.1080/15592324.2019.1665455
Seliger HH, Carpenter JH, Loftus M, McElroy WD (1970) Mechanisms for the accumulation of high concentrations of dinoflagellates in a bioluminescent bay. Limnol Oceanogr 15:234–245. https://doi.org/10.4319/lo.1970.15.2.0234
Seliger HH (1989) Mechanisms for red tides of Pyrodinium bahamense var. compressum in Papua New Guinea, Sabah and Brunei Darussalam. In: Gustaaf MH, Jay LM (eds) Biology, Epidemiology and Management of Pyrodinium Red Tides ICLARM Conf. Proc, pp 53–72
Shabbir Z, Sarosh A, Nayyer M (2019) Space Technology Applications for Maritime Intelligence, Surveillance, and Reconnaissance. Astropolitics 17:104–126. https://doi.org/10.1080/14777622.2019.1636634
Shimomura O (2006) Bioluminescence: chemical principles and methods. World Scientific Publishing Co, Pte. Ltd, Toh Tuck Link 500. https://doi.org/10.1142/6102
Shuyler LR, Linker LC, Walters CP (1995) The Chesapeake Bay story: the science behind the program. Water Sci Technol 31:133–139. https://doi.org/10.2166/wst.1995.0279
Soyer MO (1970) Les ultrastructures liées aux fonctions de relation chez Noctiluca miliaris S. (Dinoflagellata). Z Zellforsch 104:29–55. https://doi.org/10.1007/bf00340048
Stauber JL, Binet MT, Bao VW, Boge J, Zhang AQ, Leung KM, Adams MS (2008) Comparison of the qwiklite™ algal bioluminescence test with marine algal growth rate inhibition bioassays. Environ Toxicol 23:617–625. https://doi.org/10.1002/tox.20400
Stauffer BA, Gellene AG, Rico D, Sur C, Caron DA (2017) Grazing of the heterotrophic dinoflagellate Noctiluca scintillans on dinoflagellate and raphidophyte prey. Aqua Micro Ecol 80:193–207
Suescún-Bolívar LP, Thomé PE (2015) Osmosensing and osmoregulation in unicellular eukaryotes. World J Microbiol Biotechnol 31:435–443. https://doi.org/10.1007/s11274-015-1811-8
Sullivan JM, Swift E (1994) Photoinhibition of mechanically stimulable bioluminescence in the autotrophic dinoflagellate Ceratium fusus (Pyrrophyta). J Phycol 30:627–633. https://doi.org/10.1111/j.0022-3646.1994.00627.x
Sweeney BM (1971) Laboratory studies of a green Noctiluca from new guinea. J Phycol 7:53–58. https://doi.org/10.1111/j.1529-8817.1971.tb01478.x
Sweeney BM (1981) Variations of the bioluminescence per cell in Dinoflagellates. In: Nealson KH (ed) Bioluminescence Current Perspectives. Burgess Publishing, Minneapolis, pp 90–94
Sweeney BM (1986) The loss of the circadian rhythm in photosynthesis in an old strain of Gonyaulax polyedra. Plant Physiol 80:978–981. https://doi.org/10.1104/pp.80.4.978
Sweeney BM, Fork DC, Satoh K (1983) Stimulation of bioluminescence in dinoflagellates by red light. Photochemi Photobiol 37:457–465. https://doi.org/10.1111/j.1751-1097.1983.tb04499.x
Talley LD (2002) Salinity patterns in the ocean. In: MacCracken MC, Perry JS (eds) Encyclopedia of Global Change, Volume: the Earth System: Physical and Chemical Dimensions of Global Environmental Change. JohnWiley & Sons, Chichester, pp 629–640
Temnykh AV, Silakov MI, Melnik AV (2022) Large luminous plankton in bioluminescence peaks in the Black Sea. Russ J Mar Biol 48:247–255. https://doi.org/10.1134/s1063074022040113
Tillmann U, Hoppenrath M (2013) Life cycle of the pseudocolonial dinoflagellate Polykrikos kofoidii (Gymnodiniales, Dinoflagellata). J Phycol 49:298–317. https://doi.org/10.1111/jpy.12037
Trask PD (1936) Relation of salinity to the calcium carbonate content of marine sediments. U.S.Geological survey professional paper, Washington, p 279. https://doi.org/10.3133/pp186n
Tukey JW (1949) Comparing individual means in the analysis of variance. Biometrics 5:99–114. https://doi.org/10.2307/3001913
Urquhart EA, Zaitchik BF, Hoffman MJ, Guikema SD, Geiger EF (2012) Remotely sensed estimates of surface salinity in the Chesapeake Bay: a statistical approach. Remote Sens Environ 123:522–531. https://doi.org/10.1016/j.rse.2012.04.008
Valiadi M, Iglesias-Rodriguez MD (2013) Understanding bioluminescence in dinoflagellates: how far have we come? Microorganisms 1:3–25. https://doi.org/10.3390/microorganisms1010003
Valiadi M, de Rond T, Amorim A, Gittins JR, Gubili C, Moore BS, Iglesias-Rodriguez MD, Latz MI (2019) Molecular and biochemical basis for the loss of bioluminescence in the dinoflagellate Noctiluca scintillans along the west coast of the USA. Limnol Oceanogr 64:2709–2724. https://doi.org/10.1002/lno.11309
von Dassow P, Latz MI (2002) The role of Ca2+ in stimulated bioluminescence of the dinoflagellate Lingulodinium polyedrum. J Exp Biol 205:2971–2986. https://doi.org/10.1242/jeb.205.19.2971
Wegmann K (1986) Osmoregulation in eukaryotic algae. FEMS Microbiol Rev 2:37–43. https://doi.org/10.1111/j.1574-6968.1986.tb01840.x
White HH (1979) Effects of dinoflagellate bioluminescence on the ingestion rates of herbivorous zooplankton. J Exp Mar Biol Ecol 36:217–224. https://doi.org/10.1016/0022-0981(79)90117-5
Widder EA (2010) Bioluminescence in the ocean: origins of biological, chemical, and ecological diversity. Science 328:704–708. https://doi.org/10.1126/science.1174269
Wilson T, Hastings JW (2013) Bioluminescence: living lights, lights for living. Harvard University Press, Cambridge, p 185
Yoo YD, Jeong HJ, Kim JS, Kim TH, Kim JH, Seong KA, Kang NS, Park JW, Park JY, Yoon EY, Yih WH (2013) Red tides in Masan Bay, Korea in 2004–2005: II. Daily variations in the abundance of heterotrophic protists and their grazing impact on red-tide organisms. Harmful Algae 30:S89–S101. https://doi.org/10.1016/j.hal.2013.10.009
Zhang S, Liu H, Guo C, Harrison PJ (2016) Differential feeding and growth of Noctiluca scintillans on monospecific and mixed diets. Mar Ecol Prog Ser 549:27–40. https://doi.org/10.3354/meps11702
Acknowledgements
We would like to thank the editors and reviewers for their valuable feedback and constructive suggestions. Additionally, we extend our thanks to Eun Ji Kim for technical support.
Funding
Open Access funding enabled and organized by Seoul National University. This work was supported by the National Research Foundation (NRF) funded by the Ministry of Science and ICT (NRF-2021M3I6A1091272; NRF-2021R1A2C1093379; RS-2023-00291696) award to HJJ.
Author information
Authors and Affiliations
Contributions
SAP and HJJ designed the study conception and drafted the manuscript. SAP, JHO, HCK, JHY, SHE, MJL and YYD conducted experiments including bioluminescence intensities and growth rates measurement. SAP, JHO and HJJ conducted data analyses. All authors discussed the results.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All applicable international, national, and institutional guidelines for sampling, care, and experimental use of organisms for the study have been followed.
Consent to participate
Not applicable.
Consent to publish
All authors consent to the publication of this manuscript.
Additional information
Responsible Editor: S. Shumway.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, S.A., Jeong, H.J., Ok, J.H. et al. Effect of salinity on the bioluminescence intensity of the heterotrophic dinoflagellates Noctiluca scintillans and Polykrikos kofoidii and the autotrophic dinoflagellate Alexandrium mediterraneum. Mar Biol 171, 126 (2024). https://doi.org/10.1007/s00227-024-04440-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-024-04440-3