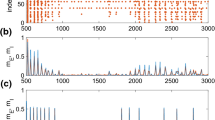
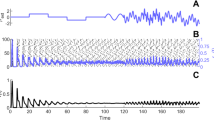
Abstract
Spiking neural systems are based on biologically inspired neural models of computation since they take into account the precise timing of spike events and therefore are suitable to analyze dynamical aspects of neuronal signal transmission. These systems gained increasing interest because they are more sophisticated than simple neuron models found in artificial neural systems; they are closer to biophysical models of neurons, synapses, and related elements and their synchronized firing of neuronal assemblies could serve the brain as a code for feature binding and pattern segmentation. The simulations are designed to exemplify certain properties of the olfactory bulb (OB) dynamics and are based on an extension of the integrate-and-fire (IF) neuron, and the idea of locally coupled excitation and inhibition cells. We introduce the background theory to making an appropriate choice of model parameters. The following two forms of connectivity offering certain computational and analytical advantages, either through symmetry or statistical properties in the study of OB dynamics have been used:
-
all-to-all coupling,
-
receptive field style coupling.
Our simulations showed that the inter-neuron transmission delay controls the size of spatial variations of the input and also smoothes the network response. Our IF extended model proves to be a useful basis from which we can study more sophisticated features as complex pattern formation, and global stability and chaos of OB dynamics.






















Similar content being viewed by others
References
Choppin PW, Pines M, Montgomery G, Goldberg J (1995) Seeing, hearing and smelling the world—new findings help scientists make sense of our senses. Howard Hughes Medical Institute, Chevy Chase
Firestein S (2001) How the olfactory system makes sense of scents. Nature 413:211–218
Gerstner W, Kistler WM (2002) Spiking neuron models, single neurons, populations, plasticity. Cambridge University Press, New York
Gordon M (1998) Shepherd, the synaptic organization of the brain. Oxford University Press, New York
Solomon E, Berg L, Martin DW (2002) Biology. Thomson Learning, Pacific Grove
Delcomyn F (1998) Foundations of neurobiology. W.H. Freeman & Company, New York
Mori K (1995) Relation of chemical structure to specificity of response in olfactory glomeruli. Curr Opin Neurobiol 5:467–474
Nagel SM, Grant LK (2002) Tutorial: the olfactory system. Athabasca University, Athabasca
Vokshoor A Anatomy of the olfactory bulb. http://www.emedicine.com/ent/topic564.htm
Mori K (1987) Membrane and synaptic properties of identified neurons in the olfactory bulb. Prog Neurobiol 29:275–320
Segev I (1999) Taming time in the olfactory bulb. Neuroscience 2:1041–1043
Urban NN (2002) Lateral inhibition in the olfactory bulb and in olfaction. Physiol Behav 77:607–612
Urban NN, Sakmann B (2002) Reciprocal intraglomerular excitation and intra- and interglomerular lateral inhibition between mouse olfactory bulb mitral cells. J Physiol 542:355–367
Davison AP, Feng J, Brown D (2000) A reduced compartmental model of the mitral cell for use in network models of the olfactory bulb. Brain Res Bull 51:393–399
Li Z, Hopfield JJ (1989) Modeling the olfactory bulb and its neural oscillatory processings. Biol Cybern 61:379–392
Hoshino O, Kashimori Y, Kambara T (1998) An olfactory recognition model based on spatio-temporal encoding of odor quality in the olfactory bulb. Biol Cybern 79:109–120
Maas W (1997) Networks of spiking neurons: the third generation of neural network models. Trans Soc Comput Simulation 14:1659–1671
Natschläger T, Maass W, Zador A (2001) Efficient temporal processing with biologically realistic dynamic synapses. Netw: Comput Neural Syst 12:75–87
Muresan RC (2003) RetinotopicNet: an efficient simulator for retinotopic visual architectures. In: European symposium on artificial neural networks. Bruges, Belgium, pp 247–254
Sterratt DC (2001) Spikes, synchrony, sequences and Schistocerca’s sense of smell, Ph.D. thesis, University of Edinburgh
Maas W, Ruf B (1996) The computational power of spiking neurons depends on the shape of the postsynaptic potentials, Electronic Colloquium on Computational Complexity (ECCC), 3
Shaw J (2004) Temporal sparse coding with spiking neurons without synfire chains, Technical Report, University of Rochester, March 2004
Li Z, Hertz J (1999) Odor recognition and segmentation by coupled olfactory bulb and cortical networks. Neurocomputing 26–27:789–794
Valova I, Gueorguieva N, Kosugi Y (2004) Oscillation-driven neural network for simulation of olfactory system. Neural Comput Appl 13(1):65–79
Kay LM, Laurent G (1999) Odor- and context-dependent modulation of mitral cell activity in behaving rats. Neuroscience 2:1003–1009
Shen GY, Chien WR, Mitdaard J, Shepard GM, Hines ML (1999) Computational analysis of action potential initiation in mitral cell soma and dendrites. J Physiol 82:3006–3020
Schoppa NE, Westbrook GL (1999) Regulation of synaptic timing in the olfactory bulb by an A-type potassium current. Neuroscience 2:1106–1113
Davison AP, Feng J, Brown D (2003) Dendrodendritic inhibition and simulated odor responses in a detailed olfactory bulb network model. J Neurophysiol 90:1921–1935
Margrie TW, Sakmann B, Urban N (2001) Action potential propagation in mitral cell lateral dendrites is decremental and controls recurrent and lateral inhibition in the mammalian olfactory bulb. PNAS 98:319–324
Maas W, Natschläger T (2001) Computing the optimally fitted spike train for a synapse. Neural Comput 13:2477–2494
Song S, Miller KD, Abbott LF (2000) Competitive Hebbian learning through spike-timing-dependent synaptic plasticity. Neuroscience 3:919–926
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valova, I., Gueorguieva, N., Troescher, F. et al. Modeling of inhibition/excitation firing in olfactory bulb through spiking neurons. Neural Comput & Applic 16, 355–372 (2007). https://doi.org/10.1007/s00521-006-0060-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00521-006-0060-z