Abstract
Purpose
The aim of this study was to assess the cost-effectiveness of NEPA, a fixed-dose combination of oral netupitant (300 mg) and palonosetron (0.5 mg), compared to available treatments in Spain after aprepitant generic introduction in the market, and to discuss results in previously performed analyses in different wordwide settings.
Methods
A Markov model including three health states, complete protection, complete response at best and incomplete response, was used to evaluate the cost-effectiveness of NEPA versus common treatment options in Spain during 5 days after chemotherapy. Incremental costs including treatment costs and treatment failure management cost as well as incremental effects including quality adjusted life days (QALDs) and emesis-free days were compared between NEPA and the comparator arms. The primary outcomes were cost per avoided emetic event and cost per QALDs gained.
Results
NEPA was dominant (more effective and less costly) against aprepitant combined with palonosetron, and fosaprepitant combined with granisetron, while, compared to generic aprepitant plus ondansetron, NEPA showed an incremental cost per avoided emetic event of €33 and cost per QALD gained of €125.
Conclusion
By most evaluations, NEPA is a dominant or cost-effective treatment alternative to current antiemetic standards of care in Spain during the first 5 days of chemotherapy treatment in cancer patients, despite the introduction of generics. These results are in line with previously reported analyses throughout different international settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is ranked by patients as one of the most distressing side effects cancer patients experience during chemotherapy [30, 42] and can negatively impact quality of life and the ability to carry out the activities of daily living [18]. Based on the Functional Living Index Emesis (FLIE) questionnaire, 37.2% of all patients reported reduced daily functioning, and of those with poorly managed CINV, about 90% reported a significant impact on daily functioning [22].
A survey among women with breast cancer showed that patients would, on average, risk a 38% chance of being dead to avoid having grade III/IV nausea/vomiting for the rest of their lives, signalling the importance of effective prophylactic treatments for these patients [30]. Experiencing CINV side effects is not only debilitating to patients but is frequently cited as a major reason for treatment discontinuation [43].
CINV is classified according to time of onset after chemotherapy administration into acute (occurring within the first 24 h), delayed (between 24 and 120 h) and overall (between 0 and 120 h) phase and may last for several days [7, 27]. Without prophylactic treatment, it is estimated that over 90% of patients exposed to highly emetogenic chemotherapy (HEC) and between 30 and 90% of patients exposed to moderately emetogenic chemotherapy (MEC) will experience acute-phase CINV [38]. Cancer drugs are classified as either low, minimal, moderate or highly emetogenic risk, based on the risk of vomiting without any antiemetic prophylaxis. Low risk is assigned to 10–30%, moderate risk to 30–90% and high risk > 90% incidence of vomiting [23]. CINV is associated with certain chemotherapy agents, e.g., HEC drugs such as cyclophosphamide (> 1500 mg/m2), cisplatin and carmustine and MEC drugs including doxorubicin, cyclophosphamide (< 1500 mg/m2), epirubicin and oxaliplatin.
While the emetogenic potential of chemotherapy agents is predictive of CINV [23], several patient-related risk factors have also been identified [26], including occurrence of CINV in previous cycle and its duration [34, 40].
Despite the introduction of more effective antiemetics, up to 20% of cancer patients treated with HEC still suffer from moderate to severe CINV (≥ grade 2) [14]. Other analysis showed, for patients receiving MEC, that despite the use of antiemetic prophylaxis, 20.8% of patients experienced at least one episode of vomiting, 42% nausea of any intensity and significant nausea in 23.8% of the patients [16].
Poor adherence to recommended prophylaxis has been reported, in several observational studies [4–6, 17]. Suboptimal adherence to prophylaxis may lead to uncontrolled CINV [28] with significant impact not only on patient’s quality of life but also on CINV-related direct costs such as acquisition cost of antiemetic drugs and rescue medication, administration devices, add-on treatments, nursing and physician time, unscheduled office visits, emergency room admissions and, in some cases, extended hospitalization or readmission [25, 35, 41]; this leads to an increased economic burden to healthcare systems, as shown in various international studies.
A study conducted in the USA describing why patients with cancer use the emergency department (ED) identified nausea and vomiting as one of the main reasons for ED visits. Of 37,760 visits, 2543 were attributable to nausea and vomiting [33].
One retrospective study analysed costs associated with CINV in patients with cancer, treated with HEC, MEC, or LEC in the outpatient setting [15]. The cost of inpatient, outpatient and ER visits and pharmacy costs (rescue medications for CINV treatment) were included. Despite prophylactic treatment, in the follow-up period, a total of 47,988 CINV events occurred with an associated all-cause treatment cost of US $89 million. The average daily CINV treatment cost for all care settings was US $1855.
A retrospective claims analysis found that patients treated with chemotherapy that experienced CINV had significantly higher CINV-related costs compared to those without CINV ($2058 and $139, respectively). Furthermore, those patients with CINV-related claims had significantly higher total direct healthcare costs compared to those with no CINV-related claims [29].
Burke et al. analysed 19,139 patients treated at 257 outpatient hospital facilities in the USA who had received HEC (16%) or MEC (84%) [12]. All patients received at least one antiemetic agent at the chemotherapy administration visit (the most common antiemetic therapies listed were 5-HT3 antagonists and corticosteroids). Approximately one in eight patients had a follow-up hospital visit associated with CINV after a first cycle of HEC or MEC. A total of 2641 patients (13.8%) experienced one or more CINV-associated hospital visit after a first cycle of HEC or MEC. Inpatient admissions (64%) were the most common type of hospital visit and were also the most costly type of visit, averaging approximately US$7500 per patient.
From a healthcare payer’s perspective, there is a need to ensure adherence to guidelines [38].
Multinational Association of Supportive Care in Cancer/European Society for Medical Oncology (MASCC/ESMO) antiemetic guidelines recommend a triplet prophylaxis with a neurokinin-1 receptor antagonist (NK1 RA), a 5-hydroxytryptamine-3 receptor antagonist (5-HT3 RA) and dexamethasone for patients receiving HEC, including anthracycline-cyclophosphamide (AC) and carboplatin (considered MEC) based chemotherapy [1, 7]. Olanzapine may be added to the triplet when the occurence of nausea associated with HEC and AC regimens is an issue [7].
Adding more liberally an NK1RA according to international guidelines’ recommendations suggests a potential reduction of healthcare resource consumption due to uncontrolled CINV.
The aim of this study was to assess the cost-effectiveness of NEPA, compared to available treatments in Spain for patients receiving HEC and to discuss results in previously performed analyses in different wordwide settings.
Methods
The targeted patient population for the analysis was cancer patients receiving prophylactic antiemetics for the management of HEC. A Markov model, previously developed for the UK [13], was adapted for Spain [15]. A 5‐day time horizon was adopted, consisting of the first day (acute phase) and a delayed phase (days 2–5), and was run for one cycle of chemotherapy (Fig. 1) [15]. Three health states were considered: complete protection (CP), complete response at best (CR) and incomplete response (no CR). Complete protection indicates no emetic episodes, no use of rescue medication and no more than mild nausea [defined as visual analogue scale < 25 mm]. Complete response was defined as no vomiting and no use of rescue medication (with studies not defining CR in this way excluded). Incomplete response indicates experience of emesis episodes and/or rescue medications.
Markov model illustrating the three health states: complete protection (CP), complete response at best (CR) and incomplete response (no CR). Note: Complete protection indicates less than 25 mm on VAS (no significant/mild nausea) without emesis and rescue medication. Complete response at best indicates at 25 mm or more on VAS without emesis and rescue medication. Incomplete response indicates experience of emesis episodes and/or rescue medications
Patients enter the economic model on the day of chemotherapy administration. Depending on the efficacy of the administered antiemetic, patients will have different probabilities of avoiding emesis and rescue medication (CR in acute phase) or experiencing emesis and/or rescue medication (no CR in acute phase). From the second to the fifth day after chemotherapy, patients will be exposed to different probabilities of avoiding emesis and rescue medication (CR in delayed phase), as opposed to failing to achieve response (no CR in delayed phase). At the end of the cycle, the average cumulative costs and effects (quality of life) will be calculated for a given treatment arm of the model. During modelling, an episode of no CR was assumed to have a large impact on costs and quality of life. In the CP health states, it was assumed a zero cost for managing an emetic episode. The analysis was evaluated from the Spanish healthcare payers´ perspective.
The efficacy data for NEPA was derived from the registration phase III trial (NETU-07–07) [24] whose patient characteristics are reported in Table 1. The efficacy for comparators were odds ratios compared to NEPA obtained from an independent network meta-analysis performed by Abdel-Rahman et al. (Table 2) [8].
The effect measures the quality of life included in the model as utilities. The utility weight is a scale from zero to one where zero is the lowest health possibly and one is perfect health. Utilities of 0.90 (95% CI 0.68–1.00) [9], 0.70 (95% CI 0.53–0.88) [9] and 0.27 (95% CI 0.18–0.30) [21] were used for CP, CR and no CR, respectively.
In the analyses, NEPA was compared with aprepitant (PO) plus ondansetron (PO), aprepitant (PO) plus palonosetron (IV), fosaprepitant (IV) plus granisetron (IV), palononostron (IV) and ondansetron (PO), all in combination with dexamethasone selected as relevant comparators on the Spanish market as well as recommended by clinical guidelines [1].
Healthcare resource utilization, i.e., proportion of patients per chemotherapy cycle including hospitalization, rescue medication, outpatient care and physician care, due to CINV, were obtained from a German-published survey including 208 patients undergoing HEC [25].
Direct costs were related to antiemetic drugs and CINV episode management, the latest being estimated from the work by Restelli et al. in an Italian setting, as no specific Spanish data were available [39]. This approach was also used in the previous adaptations for Greece and Germany [11]. Cost per hospitalization, rescue medication and physician visit were €290.31, €13.80 and €21.97, respectively. No cost for outpatient care was considered. Accordingly, the cost of CINV episode management by cycle of chemotherapy was estimated at €31.51 per patient. Drug costs were based on recommended doses from international guidelines and Spanish unit costs (ex-factory price) [2]. Generic prices, including aprepitant and fosaprepitant, were used (Table 3), and all costs were presented in 2020 euros.
Treatment-related adverse events (TRAEs) were not included in the analysis since no clinical or statistical significant differences in CINV TRAE between NEPA and the comparators were reported in the NETU trials [3, 20, 24].
The primary outcomes were incremental cost-effectiveness ratios (ICERs) for the cost per emetic event avoided and cost per quality-adjusted life days (QALDs) gained. The ICERs were calculated by dividing the difference in total costs (incremental costs) and the difference in effects (incremental emetic events or QALDs) between NEPA and the comparator treatment.
Deterministic one-way sensitivity analyses were conducted including the cost for NEPA (± 25%); cost of episode management (± 25%); utility values (95% CI); NEPA CR and CP rates in acute and overall phases (95% CI; Table 2), and odds ratios (95% CI; Table 2).
Results
Overall treatment costs with NEPA equalled to €65.40 over the 5-day time horizon with lower total treatment costs obtained with the combination of aprepitant plus ondansetron, equalled to €46.07 (Table 4). The total costs for aprepitant plus palonosetron and fosaprepitant plus granisetron were higher with €78.92 and €95.03, respectively, primarily since these treatment combinations are requiring additional IV administration costs. For CINV, episode management cost and cost for the acute and delayed phases, NEPA accrued the lowest costs among the comparators. The differences in accumulated QALDs were low between the treatments ranging from 4.117 days for aprepitant plus ondansetron to 4.272 days for NEPA. Patients in the NEPA arm were predicted to have more emesis-free and CINV-free days than the comparators (Table 3).
In summary, the results of the analysis showed that NEPA was dominant against aprepitant combined with palonosetron and fosaprepitant combined with granisetron (Table 4), while compared to generic aprepitant plus ondansetron, NEPA leads to an incremental cost per avoided emetic event of €33 and cost per QALD gained of €125.
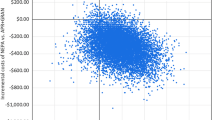
In the one-way sensitivity analysis, the CR rate for NEPA in the overall phase was found to be the most influential parameter followed by the cost of NEPA (Fig. 2).
Discussion
The results of the base case analysis showed that NEPA was dominant against aprepitant plus palonosetron and fosaprepitant plus granisetron and cost-effective against aprepitant plus ondansetron, with an ICER per cost per avoided emetic event of €33 and cost per QALDs gained of €125. Price of generic aprepitant and fosaprepitant was used in the analysis. The small QALD gained estimates in the analyses may be explained by the transient nature of CINV, and the fact that treatments were not assumed to have an impact on survival.
Beside the input of aprepitant and fosaprepitant generic prices, the results presented for Spain were in line with the results previously presented for Germany, Greece [11] and Singapore [31]. In Germany, NEPA was dominant against all comparators (i.e., aprepitant plus ondansetron, aprepitant plus palonosetron, fosaprepitant plus granisetron and rolapitant plus granisetron), being the cheapest with a total cost of €81.49 and the most effective with total QALDs of 4.272 [11]. NEPA was also dominant against all comparators in Greece (aprepitant plus ondansetron, aprepitant plus palonosetron and fosaprepitant plus granisetron), with a total cost of €85.00. Therefore, it was concluded that NEPA was a cost-effective strategy for prevention of CINV in patients undergoing HEC in both Germany and Greece. In Singapore, the results of the analysis showed that NEPA was dominant against aprepitant plus ondansetron, aprepitant plus palonosetron and fosaprepitant plus granisetron and palonosetron, and cost-effective against ondansetron, with an ICER of 47 SGD (€35) per avoided emetic event and 53,244 SGD (€40,073) per QALY gained [31].
Comparing the results from different studies poses various challenges, as depicted in CINV cost studies performed in Europe [32, 43] and the USA [12]. A study assessed the direct costs of CINV in three European countries by means of a survey covering the management and resource utilization, from patients experiencing a CINV episode during the 6-month period preceding the survey [43]. The mean cost per patient per severe CINV episode resulted in approximately €389 in Italy, €750 in France and €1017 in Germany [43]. A study from the USA reported a mean (standard deviation) cost of CINV visits of $5299 ($6639); for inpatient, $7448 ($7271); outpatient, $1494 ($2172); and emergency room, $918 ($1071) and the mean per-patient CINV-associated costs across all patients were $731 ($3069) [12]. The higher cost, presented in this study, can be explained by the fact that costs were included from the chemotherapy administration date to 30 days later, while our study was restricted to the first 5 days. Similar to the current study, Guiliani et al. [19] concluded that NEPA plus dexamethasone was cost-effective in HEC and MEC in a trial-based study. Botteman et al. [10] conducted a cost-effectiveness study using patient-level data from a phase III trial and concluded that NEPA was highly cost-effective versus an aprepitant-based regimen in post-HEC prevention. Finally, consistent results were shown also in the US setting where Park et al. showed that beside increase in acquisition cost, the introduction of NEPA into a US formulary would lead to a net decrease in the total budget due to substantial reduction in CINV event-related resource utilzation and medical cost savings for the healthcare payers [36].
The study presents several limitations. Age and sex are known risk factors for occurrence of CINV [37]. However, it was assumed that the differences in age and sex had no impact on efficacy and cost given that the target population was the general population receiving HEC for cancer treatments. Further, no Spanish data for the healthcare resource utilisation was available that may suggest an underestimation of the clinical and economic burden of CINV. The meta-analysis used for estimating comparator’s efficacy contained only odds ratios in the overall phase setting and for CR; hence, assumptions of similar differences between the acute and overall phase, as well as equal efficacy between comparators and NEPA in CP, had to be made. Finally, broad credible intervals were presented for the odds ratios in the meta-analysis possibly due to the presence of some degree of heterogeniety and selection bias of studies [8], and therefore, the mean values with 95% confidence intervals were used instead.
Conclusion
In agreement with previously published cost-effectiveness and budget impact analyses in several countries, this economic evaluation demonstrates that NEPA is a dominant or cost-effective treatment alternative by most calculations to current antiemetic standards of care in Spain during 5 days of chemotherapy treatment in cancer patients.
Data availability
Not applicable.
Code availability
Not applicable.
References
Roila F, Molassiotis A, Herrstedt J, Aapro M, Gralla RJ, Bruera E, Clark-Snow RA, Dupuis LL, Einhorn LH, Feyer P, Hesketh PJ, Jordan K, Olver I, Rapoport BL, Roscoe J, Ruhlmann CH, Walsh D, Warr D, van der Wetering M (2016) MASCC and ESMO guideline update or the prevention of chemotherapy- and radiotherapy- induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 27(suppl 5):v119–v133
Base de Datos de medicamentos del Consejo General de Farmacéuticos (BOTPLUS). Available at: https://botplusweb.farmaceuticos.com/
Aapro M, Karthaus Meinolf, Schwartzberg L, Rossi G, Rizzi G, Borroni ME, Palmas M, Rugo H (2014) Phase 3 study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron in preventing chemotherapy-induced nausea and vomiting (CINV) during repeated moderately emetogenic chemotherapy (MEC) cycles. Ann Oncol 25:1328–1333
Aapro M, Molassiotis A, Dicato M et al (2012) The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER). Ann Oncol. 23(8):1986–1992
Aapro M, Chrapava M, Curca RO et al (2020) Assessing the impact of antiemetic guideline compliance on prevention of chemotherapy-induced nausea and vomiting (CINV): results of the Results of the Nausea/Emesis Registry in Oncology (NERO). J Clin Oncol 38(15):12083
Aapro M, Scotte F, Escobar Y et al (2021) practice patterns for prevention of chemotherapy-induced nausea and vomiting and antiemetic guideline adherence based on real-world prescribing data. Oncologist 26(6):e1073–e1082
Aapro M, Scotté F, Escobar Y, Celio L, Berman R, Franceschetti A, Bell D, Jordan K (2021) Practice patterns for prevention of chemotherapy-induced nausea and vomiting and antiemetic guideline adherence based on real-world prescribing data. Oncologist. https://doi.org/10.1002/onco.13716
Abdel-Rahman O (2016) Neurokinin-1 inhibitors in the prevention of nausea and vomiting from highly emetogenic chemotherapy: a network meta-analysis. Ther Adv Med Oncol 8:396–406. https://doi.org/10.1177/1758834016654902
Annemans L, Strens D, Lox E, Petit C, Malonne H (2008) Cost-effectiveness analysis of aprepitant in the prevention of chemotherapy-induced nausea and vomiting in Belgium Supportive care in cancer : official journal of the Multinational Association of. Support Care Cancer 16:905–915. https://doi.org/10.1007/s00520-007-0349-1
Botteman M, Nickel K, Corman S, Turini M, Binder G (2020) Cost-effectiveness of a fixed combination of netupitant and palonosetron (NEPA) relative to aprepitant plus granisetron (APR + GRAN) for prophylaxis of chemotherapy-induced nausea and vomiting (CINV): a trial-based analysis. Support Care Cancer 28:857–866. https://doi.org/10.1007/s00520-019-04824-y
Bourhis FEJ, Ruffo P, D‘Agostino P, Turini M. (2018) Fixed combination netupitant and palonosetron is a cost-effective intervention for the prevention of chemotherapy-induced nausea and vomiting in Germany and Greece Spain. , ISPOR, Barcelona
Burke TA, Wisniewski T, Ernst FR (2011) Resource utilization and costs associated with chemotherapy-induced nausea and vomiting (CINV) following highly or moderately emetogenic chemotherapy administered in the US outpatient hospital setting. Support Care Cancer 19:131–140. https://doi.org/10.1007/s00520-009-0797-x
Cawston H, Bourhis F, Eriksson J, Ruffo P, D’Agostino P, Turini M, Schwartzberg L, McGuire A (2017) NEPA, a new fixed combination of netupitant and palonosetron, is a cost-effective intervention for the prevention of chemotherapy-induced nausea and vomiting in the UK. Drugs Context. 6:212298. https://doi.org/10.7573/dic.212298
Cohen L, de Moor CA, Eisenberg P, Ming EE, Hu H (2007) Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings Supportive care in cancer : official journal of the Multinational Association of. Support Care Cancer 15:497–503. https://doi.org/10.1007/s00520-006-0173-z
Craver C et al (2011) Clinical and economic burden of chemotherapy-induced nausea and vomiting among patients with cancer in a hospital outpatient setting in the United States. J Med Econ 14(1):87–98
Escobar Y, Cajaraville G, Virizuela JA, Álvarez R, Muñoz A, Olariaga O, Tamés MJ, Muros B, Lecumberri MJ, Feliu J, Martínez P, Adansa JC, Martínez MJ, López R, Blasco A, Gascón P, Calvo V, Luna P, Montalar J, Del Barrio P, Tornamira MV (2015) Incidence of chemotherapy-induced nausea and vomiting with moderately emetogenic chemotherapy: ADVICE (Actual Data of Vomiting Incidence by Chemotherapy Evaluation) study. Support Care Cancer 23:2833–2840. https://doi.org/10.1007/s00520-015-2809-3
Escobar Álvarez Y, De Castro Carpeño J, Bell D, Drago A, Franceschetti A (2021) Prevention of chemotherapy-induced nausea and vomiting in the real-world setting in Spain. Clin Translat Oncol https://doi.org/10.1007/s12094-021-02623-8
Fernández-Ortega P, Caloto MT, Chirveches E, Marquilles R, Francisco JS, Quesada A, Suárez C, Zorrilla I, Gómez J, Zabaleta P, Nocea G, Llombart-Cussac A (2012) Chemotherapy-induced nausea and vomiting in clinical practice: impact on patients’ quality of life. Support Care Cancer 20:3141–3148. https://doi.org/10.1007/s00520-012-1448-1
Giuliani I BA (2019) Netupitant plus palonosetron (NEPA) for the prophylaxis of chemotherpy-induced nausea and vomiting (CINV) in highly and moderately (AC-based chemotherapy) emetogenic cancer treatment: a cost-effective choice, June 21–23, San Fransisco, California, USA, MASCC Annual conference
Gralla RJ, Bosnjak SM, Hontsa A, Balser C, Rizzi G, Rossi G, Borroni ME, Jordan K (2014) A phase III study evaluating the safety and efficacy of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting over repeated cycles of chemotherapy. Ann Oncol 25:1333–1339. https://doi.org/10.1093/annonc/mdu096
Grunberg SM, Boutin N, Ireland A, Miner S, Silveira J, Ashikaga T (1996) Impact of nausea/vomiting on quality of life as a visual analogue scale-derived utility score. Support Care Cancer 4:435–439. https://doi.org/10.1007/bf01880641
Haiderali A, Menditto L, Good M, Teitelbaum A, Wegner J (2011) Impact on daily functioning and indirect/direct costs associated with chemotherapy-induced nausea and vomiting (CINV) in a U.S. population. Support Care Cancer 19:843–851. https://doi.org/10.1007/s00520-010-0915-9
Hesketh PJ, Kris MG, Grunberg SM, Beck T, Hainsworth JD, Harker G, Aapro MS, Gandara D, Lindley CM (1997) Proposal for classifying the acute emetogenicity of cancer chemotherapy Journal of clinical oncology : official journal of the American Society of. Clin Oncol 15:103–109. https://doi.org/10.1200/jco.1997.15.1.103
Hesketh P RG, Palmas M, Alyasova A, Gralla R. (2013) Efficacy of NEPA, a novel combination of netupitant (NETU) and palonosetron (PALO), for prevenention of chemotherapy-induced nausea and vomiting (CINV) following highly emetogenenic chemotherapy (HEC), Chicago, Illionis, USA. ASCO Annual meeting
Ihbe-Heffinger A, Ehlken B, Bernard R, Berger K, Peschel C, Eichler HG, Deuson R, Thödtmann J, Lordick F (2004) The impact of delayed chemotherapy-induced nausea and vomiting on patients, health resource utilization and costs in German cancer centers. Ann Oncol 15:526–536. https://doi.org/10.1093/annonc/mdh110
Janelsins MC, Tejani MA, Kamen C, Peoples AR, Mustian KM, Morrow GR (2013) Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin Pharmacother 14:757–766. https://doi.org/10.1517/14656566.2013.776541
Jordan K, Gralla R, Jahn F, Molassiotis A (2014) International antiemetic guidelines on chemotherapy induced nausea and vomiting (CINV): content and implementation in daily routine practice. Eur J Pharmacol 722:197–202. https://doi.org/10.1016/j.ejphar.2013.09.073
Karthaus M, Oskay-Özcelik G, Wülfing P, Hielscher C, Guth D, Zahn MO, Flahaut E, Schilling J (2020) Real-world evidence of NEPA, netupitant-palonosetron, in chemotherapy-induced nausea and vomiting prevention: effects on quality of life. Future Oncol (London, England) 16:939–953. https://doi.org/10.2217/fon-2020-0187
Knoth RL et al (2011) Health care costs in chemotherapy induced nausea and vomiting: a retrospective analysis of a commercially insured U.S. patient population. Support Care Cancer 19(Suppl 2):458
Kuchuk I, Bouganim N, Beusterien K, Grinspan J, Vandermeer L, Gertler S, Dent SF, Song X, Segal R, Mazzarello S, Crawley F, Dranitsaris G, Clemons M (2013) Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res Treat 142:101–107. https://doi.org/10.1007/s10549-013-2727-3
Lim SL, Loh KWJ, Boisseau S, Ho WT, Qasuri M, D’Agostino P, Turini M, Bourhis F, Eriksson J, Hadjiat Y (2019) Netupitant and palonosetron (NEPA) is a cost-effective intervention for the prevention of chemotherapy-induced nausea and vomiting (CINV) in Singapore. ISPOR, Copenhagen
Lordick F, Ehlken B, Ihbe-Heffinger A, Berger K, Krobot KJ, Pellissier J, Davies G, Deuson R (2007) Health outcomes and cost-effectiveness of aprepitant in outpatients receiving antiemetic prophylaxis for highly emetogenic chemotherapy in Germany. Eur J Cancer (Oxford England: 1990) 43:299–307. https://doi.org/10.1016/j.ejca.2006.09.019
Mayer DK et al (2011) Why do patients with cancer visit emergency departments? Results of a 2008 population study in North Carolina. J Clin Oncol 29(19):2683–2688
Chemotherapy-induced nausea and vomiting (CINV) duration as a predictor of later-cycle CINV Rudolph M Navari, Gary Binder, Eric J Roeland, Alexander Molassiotis, Kathryn J. Ruddy, Thomas W LeBlanc, Dwight Kloth, Silvia Sebastiani, PhD8, Marco Turini, Ravi Potluri, Luke M Schmerold, Xing Liu, Lee Schwartzberg. SASC Dec -11 virtual meeting PS13-27 https://www.abstractsonline.com/pp8/#!/9223/presentation/1480
Navari RM, Ruddy KJ, LeBlanc TW, Nipp R, Clark-Snow R, Schwartzberg L, Binder G, Bailey WL, Potluri R, Schmerold LM, Papademetriou E, Roeland EJ (2021) Avoidable acute care use associated with nausea and vomiting among patients receiving highly emetogenic chemotherapy or oxaliplatin. Oncologist 26:325–331. https://doi.org/10.1002/onco.13620
Park SH, Binder G, Corman S, Botteman M (2019) Budget impact of netupitant/palonosetron for the prevention of chemotherapy-induced nausea and vomiting. J Med Econ 22:840–847. https://doi.org/10.1080/13696998.2019.1620244
Pollera CF, Giannarelli D (1989) Prognostic factors influencing cisplatin-induced emesis Definition and validation of a predictive logistic model. Cancer 64:1117–1122. https://doi.org/10.1002/1097-0142(19890901)64:5%3c1117::aid-cncr2820640525%3e3.0.co;2-r
Rapoport BL (2017) Delayed chemotherapy-induced nausea and vomiting: pathogenesis, incidence, and current management. Front Pharmacol 8:19. https://doi.org/10.3389/fphar.2017.00019
Restelli U, Saibene G, Nardulli P, Di Turi R, Bonizzoni E, Scolari F, Perrone T, Croce D, Celio L (2017) Cost-utility and budget impact analyses of the use of NEPA for chemotherapy-induced nausea and vomiting prophylaxis in Italy. BMJ Open 7:e015645. https://doi.org/10.1136/bmjopen-2016-015645
Schwartzberg LN RM, Ruddy KJ, LeBlanc TW, Clark-Snow RA, Wickham RS, Binde RG, Bailey W, Turini M, Potluri RC, Schmerold LM, Roeland E (2020) Work loss and activity impairment due to duration of nausea and vomiting in patients with breast cancer receiving CINV Prophylaxis, Virtual, May 29–31 ASCO Annual meeting
Stewart DJ, Dahrouge S, Coyle D, Evans WK (1999) Costs of treating and preventing nausea and vomiting in patients receiving chemotherapy. J Clin Oncol 17:344–351. https://doi.org/10.1200/jco.1999.17.1.344
Sun CC, Bodurka DC, Weaver CB, Rasu R, Wolf JK, Bevers MW, Smith JA, Wharton JT, Rubenstein EB (2005) Rankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancer Supportive care in cancer : official journal of the Multinational Association of. Support Care Cancer 13:219–227. https://doi.org/10.1007/s00520-004-0710-6
Turini M, Piovesana V, Ruffo P, Ripellino C, Cataldo N (2015) An assessment of chemotherapy-induced nausea and vomiting direct costs in three EU countries. Drugs Context 4:212285. https://doi.org/10.7573/dic.212285
Funding
Helsinn Healthcare SA funded this study.
Author information
Authors and Affiliations
Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. All authors had full access to all data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. No potential author has been excluded/omitted from the authorship. All authors read an approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
Jonas Nilsson and Jennifer Eriksson are employees of Icon plc. Icon plc. received funding from Helsinn Healthcare SA for study design, execution, analysis and manuscript development. Vittoria Piovesana and Marco Turini are employees of Helsinn Healthcare SA. Helsinn Healthcare commercializes AKYNZEO® (netupitant palonosetron) worldwide. AKYNZEO® (netupitant palonosetron) is the subject of the present economic analysis. Matti Aapro has been a consultant for many companies developing antiemetics, including Helsinn.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nilsson, J., Piovesana, V., Turini, M. et al. Cost-effectiveness analysis of NEPA, a fixed-dose combination of netupitant and palonosetron, for the prevention of highly emetogenic chemotherapy-induced nausea and vomiting: an international perspective. Support Care Cancer 30, 9307–9315 (2022). https://doi.org/10.1007/s00520-022-07339-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07339-1