Abstract
Background
Patients with active cancer have a 4–sevenfold increased risk for venous thromboembolism (VTE) especially during systematic anticancer treatment. Simultaneously, surgery is an additional risk factor.
Methods
The Metaxas’s Hospital THromboprophylaxis program in Oncological & Surgical Patients (MeTHOS) is a prospective, phase IV, observational, non-interventional cohort study, aiming to record the thromboprophylaxis practice patterns in high-risk active cancer patients undergoing surgical and/or chemotherapy treatment.
Results
We are reporting results from 291 ambulatory patients (median age: 67 years, Q1–Q3: 59–73 years, 54.6% males) who received anti-neoplastic treatment and administered thromboprophylaxis. 59.8% had cardiovascular disease (mostly hypertension), 76.6% were reported as having at least one comorbidity, while 27.5% and 15.8% accumulated two and three comorbidities, respectively. 94.9% of the patients were receiving highly thrombogenic agents such as platinum-based agents, 5-FU, immunotherapy, antiangiogenics/anti-VEGF, or erythropoietin. 26.5% of the patients were initially surgically treated. In terms of anticoagulation, all patients were treated with tinzaparin (fixed dose, 10,000 Anti-Xa IU, OD). The median anticoagulation duration was 6.2 months. Six thrombotic events were observed (2.06%, 95% CI: 0.76–4.43%): 5 were DVT, and one PE. With respect to safety, 7 bleeding events occurred (2.6%, 95% CI: 1.0–5.3%); 6 of them were minor.
Conclusions
Thromboprophylaxis with LMWH in patients with active cancer and high thrombotic burden was safe and effective. Intermediate dose of tinzaparin seems to be an appropriate agent for cancer-associated thromboprophylaxis management.
Clinical trial registration
ClinicalTrials.gov: NCT04248348.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with active cancer have a 4–sevenfold increased risk of experiencing a venous thromboembolism (VTE) which is an independent risk factor for mortality, especially during the first 4 cycles of chemotherapy, in cancer patients of all stages [1, 2]. Furthermore, the incidence of cancer-associated thromboembolism (CAT) is increasing worldwide with the growing age and cancer prevalence, the enhanced detection of incidental thrombosis through CT scan, and the greater thrombogenicity of multiagent chemotherapeutic regimens [3, 4].
Additional risk factors for VTE include tumor type, stage and extent of the malignancy, as well as, the treatment with antineoplastic agents or surgery. Patient related factors, such as comorbidities and low degree of mobility can increase the thrombogenicity potential. Moreover, laboratory parameters (e.g., hemoglobin, platelets, and leukocytes) and other biomarkers (e.g., TF, TF-microparticles, thrombin, pro-inflammatory cytokines, soluble P-selectin, D-dimer and CRP) are predictive markers for the risk of VTE in cancer patients and have been used to enhance risk stratification [5, 6].
Besides the thrombotic risk, the bleeding risk for patients with active cancer needs also to be assessed [7]. Factors including age, platelet count, renal and liver status, invasive diagnostic/surgical procedures, recent immobility, recent bleeding, the cancer type and intracranial malignancy, metastasis, and systemic anticancer treatment should be taken into consideration [8]. Major challenge constitutes the management of thrombotic and bleeding risk in patients being under anticoagulation. Moreover, the bleeding risk from gastrointestinal (GI) tumors or genitourinary (GU) sites (e.g., nephrostomy tubes) should be taken into account when choosing anticoagulant agents [8].
The issue of drug-drug interactions (DDIs) is a major concern in the management of thrombosis in patients with active cancer and complicates further the selection of the proper treatment [9]. Leeuwen et al. reported that 46% of cancer patients were exposed to at least one DDI. Furthermore, 14% of these DDIs were life-threatening or exposing to permanent damage and 84% of these DDIs were exposing to a deterioration of patient’s status and a treatment was required, highlighting the clinical impact of DDIs in cancer [10].
A diligent reassessment prior to each cancer treatment line, along with the different anticancer agents administered, can facilitate the decision for thromboprophylaxis approach, and therefore, balance the various risks [11].
This thromboprophylaxis program (Metaxas’s Hospital THromboprophylaxis program in Oncological & Surgical Patients—MeTHOS, ClinicalTrials.gov identifier: NCT04248348) has been set in order to increase healthcare professionals’ awareness on the high thrombotic burden patient’s benefit. The program has provided the frame for collecting data for high thrombotic risk factors, thromboprophylaxis safety and efficacy, facilitating the awareness. Tinzaparin was chosen as an appropriate agent meeting the study needs.
Materials and methods
Study design
The Metaxas’s Hospital THromboprophylaxis program in Oncological & Surgical Patients (MeTHOS, ClinicalTrials.gov identifier: NCT04248348) is a prospective, phase IV, observational, non-interventional cohort study, aiming to record the thromboprophylaxis practices in high-risk active cancer patients undergoing surgical and/or chemotherapy treatment. The inclusion criteria were as follows: (a) diagnosis of histological confirmed high thrombotic risk solid tumors (GI, thoracic, gynecologic, and genitourinary) undergoing surgery and/or chemotherapy, (b) age ≥ 18 years, (c) ECOG 0–2, (d) life expectancy > 6 months, (e) signed informed consent. Patients undergoing chemotherapy were managed with administration of tinzaparin (fixed dose of 0.5 ml, 10.000 Anti-Xa IU, OD). The study was conformant with Helsinki declaration and subsequent amendments and was approved by the bioethics committee of “METAXA” hospital (approval protocol number: 2394–5/2/2019). Each subject’s participation was designed to last from inclusion (enrolment visit) to the last follow-up visit by the end of systemic treatment and administration of thromboprophylaxis. The entire study was expected to last by the end of 2020. Since this was a single cohort observational study, no specific design for the number of patients was performed, instead all hospital patients meeting the enrolment criteria were eligible to participate. A flowchart showing the number of patients during each program stage is presented in Fig. 1.
Along with demographic and medical history data for each patient, cancer-related data, such as primary site, staging, metastasis status, patient performance status (PS) according to ECOG scale, anticancer and anticoagulation therapy information, surgical operation details and the use of central venous catheter, were also recorded. In relation to the main study outcomes, were recorded: (a) the number of thrombotic events, (b) the dose and duration of anti-thrombotic treatment, (c) any bleedings related to anticoagulation, and (d) patients’ adherence and compliance.
Thrombotic events were assessed by physical examination and subsequently by imaging methods [12, 13]. Bleeding events were categorized as follows: (a) major, (b) clinically relevant non-major bleeding, and (c) minor bleeding. Major bleedings were defined as clinically overt bleeding events associated with a fall in hemoglobin of 2.0 g/dL (1.24 mMol/L) or more, or leading to a transfusion of ≥2 units of packed red blood cells or whole blood. As major bleedings were also defined bleedings in a critical area or organ such as: retroperitoneal, intracranial, intraocular, intraspinal, intra-articular, pericardial, and intramuscular with compartment syndrome. Additionally, a bleeding contributing to death was categorized as major bleeding. Clinically relevant non-major bleeding was defined as overt bleeding not meeting the criteria for major bleeding but associated with medical intervention, unscheduled contact (visit or telephone call) with a physician, (temporary) cessation of study treatment, or associated with discomfort for the patient such as pain, or impairment of activities of daily life. All other bleeding events were classified as minor. In cases of disagreement for the categorization of a bleeding event, an expert meeting was established in order to have a consensus and avoid any bias and additionally to attribute the bleeding to the disease or anticoagulation.
Statistical analysis
The statistical analysis was performed within the environment of the R language software platform. Descriptive values were expressed as median and 1st–3rd quartile (Q1–Q3) range (as normality via the Kolmogorov–Smirnov was not assured) and for the categorical data using frequencies and the relevant percentages. Comparisons were made using the chi-square test (or the Fisher exact test for the cases of less than 5 expected cases in more than 25% of the contingency tale cells), and using nonparametric (Mann–Whitney U) tests for continuous variables. All tests were two sided and the significance level for all study variables was set p < 0.05. In cases of missing data, a case was either excluded from the study (if one of the primary outcomes was missing) or was used only in the part of analysis that the relevant data were available. During the first step of the statistical analysis, missing data were identified and efforts to collect them retrospectively were initiated. Moreover, patients lost to follow-up were not included; data processing as the evaluation of the primary outcomes was not possible.
Results
In total, 291 ambulatory patients with active cancer receiving anticancer treatment were analyzed in the study. Their median age was 67 years and no difference (p = 0.3939) observed in the age of males and females. The median BMI of the study population was 26 kg/m2 with women having higher BMI (p = 0.0006). The characteristics of the study population are depicted in Table 1. Women had lower PS (p = 0.0100) than men. When counting comorbidities, 76.6% were reported as having at least one comorbidity, while 4, 3, or 2 comorbidities had 7.2%, 15.8%, and 27.5% of patients, respectively.
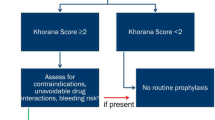
There was a varying and highly thrombotic potential of the primary cancer sites, All patients included at this study had Khorana score ≥ 2; moreover, 108 (37.1%) cases involved the gastrointestinal system (among them: colorectal 36.1%, pancreas 33.3%, gastric 25%, and other sites of the GI system 5.6%), 66 cases (22.7%) involved the lung, 47 cases (16.2%) the woman reproductive system (ovaries 63.8%, uterine 29.8%, and cervix 6.4%), 26 (8.9%) the breast, 24 (8.3%) the urinary system (bladder 45.8%, prostate 33%, and renal 20.8%), 7 cases (2.4%) were head and neck cancers, and 13 (4.5%) cases had other (or unknown) primary site. Metastatic patients comprised 72.9% of the population (see Fig. 2 for a graphical representation of the primary sites and the percentage of metastasis per site).
Concerning anticancer treatment, 97.9% of the patients were receiving HTAs such as platinum-based agents, 5-FU, erythropoietin, or immunotherapy; more details are presented in Table 2. Furthermore, 26.5% of the patients had been surgically treated and 22.5% had a central venous catheter representing additional risk factors for thrombosis. Notably, a high percentage of these highly thrombogenic agents (71.5%) had potential drug–drug interactions (DDIs) with direct oral anticoagulants (DOACs). Agents with potential DDIs were identified according to bibliographical data [14,15,16,17,18,19,20] and are reported in the Appendix table 5.
The average monitoring time from enlistment until last time a patient was seen was 148 ± 117 days. In terms of anticoagulation, in all patients was administered tinzaparin (10.000 Anti-Xa IU, OD) based on hospital protocol. The median anticoagulation duration for the study period was 6.2 months (Q1–Q3: 4.0–10.0 months). The duration was differentiated in the various cancer sites (p = 0.0621); Table 3 presents various characteristics of the patients, neoplasm details, anticancer treatment period, and anticoagulation approach in relation to cancer primary site. Notably, 15 patients with atrial fibrillation receiving oral anticoagulation switched to tinzaparin (10.000 Anti-Xa IU, OD) during the study period. In these patients, no thrombosis was reported, and 1 minor bleeding occurred.
With regard to efficacy, six thrombotic events were observed (2.06%, 95% CI: 0.76–4.43%); from these, 5 events were DVT, and one was PE. Their major characteristics are presented in Table 4. All these patients had thrombosis history or suffered from cardiovascular disease or diabetes mellitus. However, the small number of thrombotic events is not sufficient to verify any relations of such events with the aspects recorded during MeTHOS study.
With respect to safety, 7 bleeding events occurred (2.6%, 95% CI: 1.0–5.3%). Six of them were minor and one was major, in a 68-year-old woman with metastatic pancreatic cancer. BMI found to be related to bleeding (p = 0.0036), as the median BMI of the patients with bleeding was 20.0 (Q1–Q3: 18.7–25.4) while the patients that did not experienced bleeding had median BMI 26.0 (Q1–Q3: 23.0–29.4). Similarly, patients with lower body weight were more prone to bleeding (p = 0.0274); specifically, patients that experienced a bleeding event had median weight 58 kg (Q1–Q3: 53–75 kg) and the patients that did not experienced such events had median weight 71 kg (Q1–Q3: 60–83). As in the case of thrombotic events, the small number of bleeding events did not let to reveal any associations. Remarkably, 5 out of 7 bleeding events were related to the GI system and 5 out of 7 cases were men. However, no statistically significant difference can be confirmed, neither for the anatomical site nor for the gender.
Discussion
In our hospital protocol were enrolled 291 active cancer patients with high thrombotic burden, which required thromboprophylaxis with low molecular weight heparin (LMWH) and the aim was to monitor efficacy and safety of thromboprophylaxis management in those patients. According to our results, the thromboprophylaxis management with tinzaparin 10,000 Anti-Xa IU, OD found to be effective without compromising the patients’ safety.
Cancer patients are fragile, usually of older age, have a poor performance status, with comorbidities requiring polypharmacy, high incidence of renal impairment, and are exposed to treatment combinations with potentially nephrotoxic effects [21]. Of note, in the current study, cancer patients had history of numerous simultaneous comorbidities, meaning almost 4 in 5 patients were dealt with polypharmacy, which is common in cancer [22] and also in thrombosis [23] patients. Furthermore, there is a close relationship between polypharmacy and DDIs. LMWH has been the recognized standard treatment for more than a decade, both in cancer-related thrombosis treatment and prevention [24]. Direct oral anticoagulants (DOACs) are a new option for anticoagulation therapy [25] but interactions with anticancer or other supportive drugs may be challenging, while for LMWHs, there are no known interactions. All DOACs are transported by P-glycoprotein, and in addition, rivaroxaban and apixaban are substrates for cytochrome P450 (CYP3A4) [26, 27]. Many drugs used in systematic anticancer therapy are inhibitors or inducers of P-glycoprotein and/or CYP3A4, which may potentially result in a change of plasma DOAC concentration, taking it outside the therapeutic window. The result of this may be lack of a therapeutic effect or an increase in the number of bleeding complications [28].
It is known that the thrombotic risk is highest for patients with certain malignancies, including lung, GI, and GU cancers. Lung cancer (LC) comprise the 22.7% of the current population and it is known that LC is a leading cause of cancer death in the USA for both men and women [29]. It is also one of the malignancies that are commonly associated with VTE, including PE, with reported incidence of VTE up to 13.8% and that of PE up to 3.8% [30,31,32]. Recently, Zhang et al. described the high prevalence of VTE in patients with newly diagnosed LC and VTE events occurred in 89 (13.2%) of the 673 patients enrolled in the study. Forty-two (6.2%) patients developed lower extremity deep vein thrombosis (DVT) alone, 33 (4.9%) patients developed pulmonary embolism (PE), and 14 (2.1%) patients developed both DVT and PE [33]. No VTE events were reported in lung cancer patients in our cohort.
High rates of symptomatic and incidental thromboembolic events have been reported in gastrointestinal (GI) cancer patients which represent the 37.1% in the existing cohort. In a retrospective study which included a total of 220 consecutive GI cancer patients, sixty patients (27.3%) were found to have experienced a total of 83 thromboembolic events. These included 32 DVTs (38.6%) and 17 PEs (20.5%). An additional twenty three patients developed 25 (30.1%) visceral vein or 9 (10.8%) arterial thromboembolic events [34]. Only one VTE event reported in GI cancer patients in our analysis, specifically in a pancreatic cancer patient. In the present population, the 33% of the GI malignancies were patients with pancreatic cancer. Reported frequencies of thrombosis associated with pancreatic cancer are the highest compared with other malignancies. The first case series describing the striking relationship between pancreatic cancer and thrombosis was published in 1938 where it is documented a 60% prevalence of venous thromboembolism in patients with pancreatic cancer at autopsy. Studies carried out over the past 10–15 years have reported VTE prevalence rates of up to 36% in patients with pancreatic cancer [27].
Gynecologic cancer patients constituted 16.2% of the current study population and this cancer type has been also associated with high incidence of VTE in previous trials. In a retrospective study, among 1885 women with gynecologic cancer, 769 (40.8%) experienced venous thromboembolic events, most of them in the first 2 years after cancer diagnosis. Specifically, 40.4% of patients experienced DVT, while PE occurred in 1.2% of the patients. There was no statistically significant difference in the incidence of VTE according to the type of gynecologic cancer [35]. Two VTE events were reported in patients with gynecologic cancer in our cohort.
The presence of metastases is associated with increased hypercoagulability, as the hemostatic system seems to play a key role in the metastatic capacity of solid tumors [36]. Additionally, patients with metastatic disease at the time of diagnosis had up to 21.5 times higher risk for thromboembolism in comparison to patients with non-metastatic disease. Also, there is data shown that mucinous adenocarcinomas, such as pancreatic, lung, and cancers of the gastrointestinal tract, are those with the highest incidence of cancer-related VTE [37,38,39]. Cancer patients with metastatic disease comprised the 72.9% of the current study population and metastasis is considered a dominant factor for VTE. Four VTE events and two bleedings were observed in metastatic patients in our analysis. In a similar cancer population, VTE prevalence was found to increase with stage sharply in patients with tumors at a higher stage [40, 41]. Similarly, advanced disease stages and distant metastases increase VTE risk as it is shown in the Blom et al. report where an adjusted odds ratio (OR) of 19.8 for VTE risk was noticed in patients with metastatic cancer compared with patients without overt metastases [42].
Cancer therapy and thrombosis for over three decades remained an underappreciated risk that has not been routinely incorporated into thrombosis risk assessment models [43]. Mechanisms that drive cancer therapy–associated thrombosis are not fully understood. A primary mechanism may be the activation or disruption of the endothelium by anticancer agents. In addition, these agents may decrease anticoagulants and increase procoagulants, such as tissue factor (TF), leading to activation of coagulation. Finally, anticancer drugs may directly or indirectly activate platelets [44].Cancer patients undergoing systemic treatment for their malignancy are among the highest risk populations for thromboembolic complications; highly thrombogenic chemotherapy agents (HTAs) include platinum compounds, 5-FU, capecitabine, gemcitabine, hormonal therapy, anti-angiogenesis treatment, e.g., bevacizumab, and supportive treatment, e.g., corticosteroids, erythropoietin [45]. VTE is also common in cancer patients receiving immunotherapy either as single-agent or combination regimens, affecting nearly one-third of all immunotherapy treated patients and may potentially be associated with worsened survival [46]. HTAs were used at 97.9% the present population.
In a retrospective observational study [47] of 18,531 patients diagnosed with a malignant tumor, the majority of VTE events occurred shortly after the diagnosis of cancer. Among the cancer patients, 53.92% had a VTE event within the first 3 months, 63.21% within the first 6 months, and 68.93% within the first 9 months (notably patients experienced more than a single VTE event). The median duration of anticoagulation treatment during the present study period was 6.2 months representing also the median duration of antineoplastic treatment.
The LMWHs constitute standard of care along with DOACs for the treatment and prevention of VTE for patients with active cancer, without the warning for the safety considerations and DDIs that follow the DOACs [48, 49]. There is strong evidence that the coagulation plays an important role in cancer metastasis and angiogenesis [50]. The anti-tumor effect of heparins, particularly LMWH, has been confirmed. These anticoagulants inhibit cancer cell growth and metastasis formation in several ways. The anti-angiogenic effect of LMWH is found to be expressed in a dose-dependent manner [51, 52]. Tinzaparin sodium Xa inhibitory effect is dose-dependent, and higher, as compared to its anti-IIa activity [53]; moreover, it disposes the highest anti-IIa activity among all LMWHs. Since LMWHs express “pleiotropic effects” in a dose-dependent manner, a “high thrombotic burden (HTB)”-adapted strategy could help high-risk patients who may benefit beyond anticoagulation from use of higher than conventional prophylactic LMWH dose. Notably, in recently published data, intermediate-dose tinzaparin (8000–12,000 Anti‑Xa IU, once daily) was found to be more efficacious for the prevention of VTE, without compromising safety [54]. Tinzaparin sodium possesses important pharmacokinetic properties, with the consecutive involvement of cellular and renal route of elimination, exhibiting no bioaccumulation even in patients with severe renal impairment, maintaining a special stand among other LMWHs [55] [56]. With the above mentioned characteristics, tinzaparin seems that reconciles the relevant profile for patients with active cancer who combine multiple factors worsening their renal function. Such factors include but are not limited to: older age, dehydration, use of nephrotoxic agents for anticancer treatment and other comorbidities, such as hypertension and diabetes mellitus [55]. Furthermore, anticancer effects have also been delineated in vitro, in vivo, and retrospective trials [57,58,59,60].
The effect of thromboprophylaxis with other LMWHs in various solid tumors is considered in two main RCTs: SAVE ONCO [61] using semuloparin and PROTECT [62] using nadroparin. Patients’ characteristics and malignancies are comparable with current cohort. In PROTECT, the median prophylaxis duration was less than 4 months, and in SAVE ONCO, it was 3.5 months, while in our cohort, the average duration was longer than 6 months. With regard to efficacy, thromboembolic events were experienced by 2.0% of the patients treated with nadroparin in PROTECT and by 1.2% of the patients receiving semuloparin in SAVE ONCO similar to our study (2.06%).
In terms of safety, minor bleeding events occurred in 7.4% of patients treated with nadroparin in the PROTECT study, and major ones in 0.7% of them. The incidence of clinically relevant bleeding in SAVE ONCO was 2.8%, and that of major bleeding 1.2% in the semuloparin group. In the MeTHOS cohort, 7 bleeding events occurred (2.6%). Six of them were minor and one major. In both PROTECT and SAVE ONCO trials, the dose used was the prophylactic one.
Our study had the limitations and advantages of a pragmatic study [63] designed in a broad range of routine clinical practice, without specific focus on patients’ characteristics; thus, unknown bias could have been introduced. There was no selection of patients into intervention. Therefore, in the authors’ opinion, this study captured the real-life conditions in a routine clinical oncology setting. One of the strengths of our approach was the validity of our results, related to efficacy and safety due to the fact that thromboprophylaxis duration lasted 6 months.
ffffffffThe risk of VTE is increasing in patients with active cancer and the MeTHOS study demonstrates that it is important to assess the thrombotic burden in patients receiving anticancer treatment. Individuals at increased thrombotic risk should be offered thromboprophylaxis to avoid serious and life-threatening complications. The administration of LMWH (tinzaparin intermediate dose 10,000 Anti-Xa IU, OD) appears to offer an effecctive and safe solution for thrombo-prophylaxis during the course of anti-cancer treatment.
Data availability
Study data are available from the corresponding author upon reasonable request.
References
Heit JA, Spencer FA, White RH (2016) The epidemiology of venous thromboembolism. J Thromb Thrombolysis 41(1):3–14. https://doi.org/10.1007/s11239-015-1311-6
Kuderer NM, Culakova E, Lyman GH, Francis C, Falanga A, Khorana AA (2016) A validated risk score for venous thromboembolism is predictive of cancer progression and mortality. Oncologist 21(7):861–867. https://doi.org/10.1634/theoncologist.2015-0361
Khorana AA, Francis CW, Culakova E, Fisher RI, Kuderer NM, Lyman GH (2006) Thromboembolism in hospitalized neutropenic cancer patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 24(3):484–490. https://doi.org/10.1200/JCO.2005.03.8877
Mulder FI, Horvath-Puho E, van Es N, van Laarhoven HWM, Pedersen L, Moik F et al (2021) Venous thromboembolism in cancer patients: a population-based cohort study. Blood 137(14):1959–1969. https://doi.org/10.1182/blood.2020007338
Ay C, Pabinger I, Cohen AT (2017) Cancer-associated venous thromboembolism: burden, mechanisms, and management. Thromb Haemost 117(2):219–230. https://doi.org/10.1160/TH16-08-0615
Haltout J, Awada A, Paesmans M, Moreau M, Klastersky J, Machiels G et al (2019) Predictive factors for cancer-associated thrombosis in a large retrospective single-center study. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 27(4):1163–1170. https://doi.org/10.1007/s00520-018-4602-6
Frere C, Font C, Esposito F, Crichi B, Girard P, Janus N (2022) Incidence, risk factors, and management of bleeding in patients receiving anticoagulants for the treatment of cancer-associated thrombosis. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 30(4):2919–2931. https://doi.org/10.1007/s00520-021-06598-8
Patel HK, Khorana AA (2019) Anticoagulation in cancer patients: a summary of pitfalls to avoid. Curr Oncol Rep 21(2):18. https://doi.org/10.1007/s11912-019-0767-5
Min JS, Bae SK (2017) Prediction of drug-drug interaction potential using physiologically based pharmacokinetic modeling. Arch Pharmacal Res 40(12):1356–1379. https://doi.org/10.1007/s12272-017-0976-0
van Leeuwen RW, Brundel DH, Neef C, van Gelder T, Mathijssen RH, Burger DM et al (2013) Prevalence of potential drug-drug interactions in cancer patients treated with oral anticancer drugs. Br J Cancer 108(5):1071–1078. https://doi.org/10.1038/bjc.2013.48
Farmakis I, Zafeiropoulos S, Pagiantza A, Boulmpou A, Arvanitaki A, Tampaki A et al (2020) Low-density lipoprotein cholesterol target value attainment based on 2019 ESC/EAS guidelines and lipid-lowering therapy titration for patients with acute coronary syndrome. Eur J Prev Cardiol 27(19):2314–2317. https://doi.org/10.1177/2047487319891780
Estrada YMRM, Oldham SA (2011) CTPA as the gold standard for the diagnosis of pulmonary embolism. Int J Comput Assist Radiol Surg 6(4):557–563. https://doi.org/10.1007/s11548-010-0526-4
Zierler BK (2004) Ultrasonography and diagnosis of venous thromboembolism. Circulation 109(12 Suppl 1):I9-14. https://doi.org/10.1161/01.CIR.0000122870.22669.4a
Short NJ, Connors JM (2014) New oral anticoagulants and the cancer patient. Oncologist 19(1):82–93. https://doi.org/10.1634/theoncologist.2013-0239
Chu G, Versteeg HH, Verschoor AJ, Trines SA, Hemels MEW, Ay C et al (2019) Atrial fibrillation and cancer - an unexplored field in cardiovascular oncology. Blood Rev 35:59–67. https://doi.org/10.1016/j.blre.2019.03.005
Wang TF, Baumann Kreuziger L, Leader A, Spectre G, Lim MY, Gahagan A et al (2021) Characteristics and outcomes of patients on concurrent direct oral anticoagulants and targeted anticancer therapies-TacDOAC registry: communication from the ISTH SSC Subcommittee on Hemostasis and Malignancy. J Thromb Haemost 19(8):2068–2081. https://doi.org/10.1111/jth.15367
van Leeuwen RW, van Gelder T, Mathijssen RH, Jansman FG (2014) Drug-drug interactions with tyrosine-kinase inhibitors: a clinical perspective. Lancet Oncol 15(8):e315–e326. https://doi.org/10.1016/s1470-2045(13)70579-5
Hussaarts K, Veerman GDM, Jansman FGA, van Gelder T, Mathijssen RHJ, van Leeuwen RWF (2019) Clinically relevant drug interactions with multikinase inhibitors: a review. Ther Adv Med Oncol 11:1758835918818347. https://doi.org/10.1177/1758835918818347
Delord JP, Ravaud A, Bennouna J, Fumoleau P, Favrel S, Pinel MC et al (2013) Phase I and pharmacokinetic study of IV vinflunine in cancer patients with liver dysfunction. Invest New Drugs 31(3):724–733. https://doi.org/10.1007/s10637-012-9878-7
Groenland SL, Martinez-Chavez A, van Dongen MGJ, Beijnen JH, Schinkel AH, Huitema ADR et al (2020) Clinical pharmacokinetics and pharmacodynamics of the cyclin-dependent kinase 4 and 6 inhibitors palbociclib, ribociclib, and abemaciclib. Clin Pharmacokinet 59(12):1501–1520. https://doi.org/10.1007/s40262-020-00930-x
Elalamy I, Canon J, Bols A, Lybaert W, Duck L, Jochmans K et al (2014) Thrombo-embolic events in cancer patients with impaired renal function. Journal of Blood Disorders & Transfusion 5(4):1000202. https://doi.org/10.4172/2155-9864.1000202
Nightingale G, Hajjar E, Swartz K, Andrel-Sendecki J, Chapman A (2015) Evaluation of a pharmacist-led medication assessment used to identify prevalence of and associations with polypharmacy and potentially inappropriate medication use among ambulatory senior adults with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 33(13):1453–1459. https://doi.org/10.1200/JCO.2014.58.7550
Martinez BK, Baker WL, Sood NA, Bunz TJ, Meinecke AK, Eriksson D et al (2019) Influence of polypharmacy on the effectiveness and safety of rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation. Pharmacotherapy 39(2):196–203. https://doi.org/10.1002/phar.2213
Scotte F, Elalamy I, Mayeur D, Meyer G (2018) Physicians’ decision about long-term thromboprophylaxis in cancer outpatients: CAT AXIS, a case vignette study on clinical practice in France. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 26(6):2049–2056. https://doi.org/10.1007/s00520-017-4034-8
Iorga RA, Bratu OG, Marcu RD, Constantin T, Mischianu DLD, Socea B et al (2019) Venous thromboembolism in cancer patients: still looking for answers. Exp Ther Med 18(6):5026–5032. https://doi.org/10.3892/etm.2019.8019
Galgani A, Palleria C, Iannone LF, De Sarro G, Giorgi FS, Maschio M et al (2018) Pharmacokinetic interactions of clinical interest between direct oral anticoagulants and antiepileptic drugs. Front Neurol 9:1067. https://doi.org/10.3389/fneur.2018.01067
Campello E, Ilich A, Simioni P, Key NS (2019) The relationship between pancreatic cancer and hypercoagulability: a comprehensive review on epidemiological and biological issues. Br J Cancer 121(5):359–371. https://doi.org/10.1038/s41416-019-0510-x
Vazquez SR (2018) Drug-drug interactions in an era of multiple anticoagulants: a focus on clinically relevant drug interactions. Hematology Am Soc Hematol Educ Program 2018(1):339–347. https://doi.org/10.1182/asheducation-2018.1.339
Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer statistics, 2021. CA: a cancer journal for clinicians. 71(1):7–33. https://doi.org/10.3322/caac.21654
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA: a cancer journal for clinicians. 60(5):277–300. https://doi.org/10.3322/caac.20073
Tagalakis V, Levi D, Agulnik JS, Cohen V, Kasymjanova G, Small D (2007) High risk of deep vein thrombosis in patients with non-small cell lung cancer: a cohort study of 493 patients. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2(8):729–734. https://doi.org/10.1097/JTO.0b013e31811ea275
Walker AJ, Baldwin DR, Card TR, Powell HA, Hubbard RB, Grainge MJ (2016) Risk of venous thromboembolism in people with lung cancer: a cohort study using linked UK healthcare data. Br J Cancer 115(1):115–121. https://doi.org/10.1038/bjc.2016.143
Zhang Y, Yang Y, Chen W, Guo L, Liang L, Zhai Z et al (2014) Prevalence and associations of VTE in patients with newly diagnosed lung cancer. Chest 146(3):650–658. https://doi.org/10.1378/chest.13-2379
Singh R, Sousou T, Mohile S, Khorana AA (2010) High rates of symptomatic and incidental thromboembolic events in gastrointestinal cancer patients. J Thromb Haemost 8(8):1879–1881. https://doi.org/10.1111/j.1538-7836.2010.03929.x
Trugilho IA, Renni MJP, Medeiros GC, Thuler LCS, Bergmann A (2020) Incidence and factors associated with venous thromboembolism in women with gynecologic cancer. Thromb Res 185:49–54. https://doi.org/10.1016/j.thromres.2019.11.009
Gale AJ, Gordon SG (2001) Update on tumor cell procoagulant factors. Acta Haematol 106(1–2):25–32. https://doi.org/10.1159/000046586
Khorana AA, Connolly GC (2009) Assessing risk of venous thromboembolism in the patient with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 27(29):4839–4847. https://doi.org/10.1200/JCO.2009.22.3271
Blom JW, Osanto S, Rosendaal FR (2004) The risk of a venous thrombotic event in lung cancer patients: higher risk for adenocarcinoma than squamous cell carcinoma. J Thromb Haemost 2(10):1760–1765. https://doi.org/10.1111/j.1538-7836.2004.00928.x
Khorana AA, Francis CW, Culakova E, Lyman GH (2005) Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer 104(12):2822–2829. https://doi.org/10.1002/cncr.21496
Munoz Martin AJ, Ortega I, Font C, Pachon V, Castellon V, Martinez-Marin V et al (2018) Multivariable clinical-genetic risk model for predicting venous thromboembolic events in patients with cancer. Br J Cancer 118(8):1056–1061. https://doi.org/10.1038/s41416-018-0027-8
Gade IL, Braekkan SK, Naess IA, Hansen JB, Cannegieter SC, Overvad K et al (2017) The impact of initial cancer stage on the incidence of venous thromboembolism: the Scandinavian Thrombosis and Cancer (STAC) cohort. J Thromb Haemost 15(8):1567–1575. https://doi.org/10.1111/jth.13752
Blom JW, Doggen CJ, Osanto S, Rosendaal FR (2005) Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA, J Am Med Assoc 293(6):715–722. https://doi.org/10.1001/jama.293.6.715
Otten HM, Mathijssen J, ten Cate H, Soesan M, Inghels M, Richel DJ et al (2004) Symptomatic venous thromboembolism in cancer patients treated with chemotherapy: an underestimated phenomenon. Arch Intern Med 164(2):190–194. https://doi.org/10.1001/archinte.164.2.190
Grover SP, Hisada YM, Kasthuri RS, Reeves BN, Mackman N (2021) Cancer therapy-associated thrombosis. Arterioscler Thromb Vasc Biol 41(4):1291–1305. https://doi.org/10.1161/ATVBAHA.120.314378
Oppelt P, Betbadal A, Nayak L (2015) Approach to chemotherapy-associated thrombosis. Vasc Med 20(2):153–161. https://doi.org/10.1177/1358863X14568705
Roopkumar J, Kim AS, Bicky T, Hobbs BP, Khorana AA (2018) Venous thromboembolism in cancer patients receiving immunotherapy. Blood 132:2510. https://doi.org/10.1182/blood-2018-99-116439
Wang H, Xu X, Pu C, Li L (2019) Clinical characteristics and prognosis of cancer patients with venous thromboembolism. J Cancer Res Ther 15(2):344–349. https://doi.org/10.4103/jcrt.JCRT_121_18
Papakotoulas P, Tsoukalas N, Christopoulou A, Ardavanis A, Koumakis G, Papandreou C et al (2020) Management of cancer-associated thrombosis (CAT): symptomatic or incidental. Anticancer Res 40(1):305–313. https://doi.org/10.21873/anticanres.13954
Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI et al (2020) Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 38(5):496–520. https://doi.org/10.1200/JCO.19.01461
Rojas-Hernandez CM (2018) The role of direct oral anticoagulants in cancer-related venous thromboembolism: a perspective beyond the guidelines. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 26(3):711–720. https://doi.org/10.1007/s00520-017-3990-3
Zhang N, Lou W, Ji F, Qiu L, Tsang BK, Di W (2016) Low molecular weight heparin and cancer survival: clinical trials and experimental mechanisms. J Cancer Res Clin Oncol 142(8):1807–1816. https://doi.org/10.1007/s00432-016-2131-6
Bochenek J, Püsküllüoğlu M, Krzemieniecki K (2013) The antineoplastic effect of low-molecular-weight heparins - a literature review. Contemp Oncol (Pozn) 17(1):6–13. https://doi.org/10.5114/wo.2013.33766
Fossler MJ, Barrett JS, Hainer JW, Riddle JG, Ostergaard P, van der Elst E et al (2001) Pharmacodynamics of intravenous and subcutaneous tinzaparin and heparin in healthy volunteers. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists 58(17):1614–1621. https://doi.org/10.1093/ajhp/58.17.1614
Christopoulou A, Ardavanis A, Papandreou C, Koumakis G, Papatsimpas G, Papakotoulas P et al (2022) Prophylaxis of cancer-associated venous thromboembolism with low-molecular-weight heparin-tinzaparin: real world evidence. Oncol Lett 23(4):115. https://doi.org/10.3892/ol.2022.13235
Dimakakos EP, Vathiotis I, Syrigos K (2018) The role of tinzaparin in oncology. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis 24(5):697–707. https://doi.org/10.1177/1076029617729215
Scotte F, Rey JB, Launay-Vacher V (2012) Thrombosis, cancer and renal insufficiency: low molecular weight heparin at the crossroads. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 20(12):3033–3042. https://doi.org/10.1007/s00520-012-1590-9
Schlesinger M, Roblek M, Ortmann K, Naggi A, Torri G, Borsig L et al (2014) The role of VLA-4 binding for experimental melanoma metastasis and its inhibition by heparin. Thromb Res 133(5):855–862. https://doi.org/10.1016/j.thromres.2014.02.020
Alyahya R, Sudha T, Racz M, Stain SC, Mousa SA (2015) Anti-metastasis efficacy and safety of non-anticoagulant heparin derivative versus low molecular weight heparin in surgical pancreatic cancer models. Int J Oncol 46(3):1225–1231. https://doi.org/10.3892/ijo.2014.2803
Sarantis P, Bokas A, Papadimitropoulou A, Koustas E, Theocharis S, Papakotoulas P et al. (2021) Combinatorial treatment of tinzaparin and chemotherapy can induce a significant antitumor effect in pancreatic cancer. International journal of molecular sciences. 22(13). https://doi.org/10.3390/ijms22137053
Karamouzis MV, Athanasiadis I, Samelis G, Vallilas C, Bokas A, Nikolaidi A et al. (2021) The impact of thromboprophylaxis on the survival of patients with advanced pancreatic cancer. The Pancreatic Cancer and Tinzaparin (PaCT) Study. Cancers (Basel). 13(12). https://doi.org/10.3390/cancers13122884
Agnelli G, George DJ, Kakkar AK, Fisher W, Lassen MR, Mismetti P et al (2012) Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med 366(7):601–609. https://doi.org/10.1056/NEJMoa1108898
Agnelli G, Gussoni G, Bianchini C, Verso M, Mandala M, Cavanna L et al (2009) Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol 10(10):943–949. https://doi.org/10.1016/S1470-2045(09)70232-3
Patsopoulos NA (2011) A pragmatic view on pragmatic trials. Dialogues Clin Neurosci 13(2):217–224
Author information
Authors and Affiliations
Contributions
S.X. and N.Z. conceived and designed the study. C.K., N.C., and E.L. supervised patients’ treatment. D.S., G.P., E.M., and A.N.L. collected the data. A.P. performed the data analysis. All authors wrote and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Bioethics committee of “METAXA” Memorial Piraeus Cancer Hospital. The study was performed in accordance with the Declaration of Helsinki. All patients had signed an informed consent form.
Consent for publication.
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xynogalos, S., Simeonidis, D., Papageorgiou, G. et al. Can thromboprophylaxis build a link for cancer patients undergoing surgical and/or chemotherapy treatment? The MeTHOS cohort study. Support Care Cancer 30, 6973–6984 (2022). https://doi.org/10.1007/s00520-022-07096-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07096-1