Abstract
Background
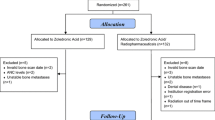
The effect of longer-term use of bone-modifying agent (BMA) on symptomatic skeletal event (SSE) rates in patients with bone metastases remains unclear. This retrospective study of a cohort of patients in a randomized controlled trial evaluated SSEs in patients receiving BMAs at a single cancer center.
Methods
Data from patients with metastatic breast and castration-resistant prostate cancer (CRPC) were interrogated to evaluate the effects of longer-term use of BMAs on incidence, type, and risk factors for SSEs.
Results
Of 162 patients, 109 (67%) had breast cancer (BC) and 53 (33%) CRPC. Median age at diagnosis of bone metastases was 61.9 years (range 27.5–97.2) for BC patients and 72.1 (range 37.0–92.2) for CRPC patients. Median duration of BMA use was 2.3 years (range 0.1–9.9 years) for BC and 3.8 years (range 1.5–9.4) for CRPC patients. The initial BMAs in BC patients were pamidronate (46.8%), denosumab (31.2%), and zoledronate (22%). All CRPC patients received denosumab. During follow-up, 59% of BC and 75% of CRPC patients had at least one SSE. The number of patients experiencing ≥ 1 SSE per year was higher in the first year after bone metastasis diagnosis (63/162; 38.9%) compared with that in the second (26/149; 17.5%) and third years (30/123; 24.4%). Neither age, visceral disease, multiple bone metastases, nor biological markers for BC had a significant impact on time to first SSE.
Conclusions
The risk for SSEs was greatest in the first year after diagnosis of bone metastasis. Studies evaluating de-escalation and even stopping of BMAs with longer-term use may therefore be warranted.


Similar content being viewed by others
Data availability
De-identified dataset is available upon request and approval by the Ontario Cancer Research Ethics Board (OCREB 1–866-678–6427 Ext 6649).
Code availability
N/A.
References
Coleman RE (1997) Skeletal complications of malignancy. Cancer 80(8 Suppl):1588–1594. https://doi.org/10.1002/(sici)1097-0142(19971015)80:8+%3c1588::aid-cncr9%3e3.3.co;2-z
Coleman RE, Rubens RD (1987) The clinical course of bone metastases from breast cancer. Br J Cancer 55(1):61–66. https://doi.org/10.1038/bjc.1987.13
Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA (2009) Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat 115(2):423–428. https://doi.org/10.1007/s10549-008-0086-2
Hernandez RK, Wade SW, Reich A, Pirolli M, Liede A, Lyman GH (2018) Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC Cancer 18(1):44. https://doi.org/10.1186/s12885-017-3922-0
Bongiovanni A, Recine F, Fausti V, Foca F, Casadei R, Falasconi MC, Oboldi D, Sansoni E, Fabbri L, Micheletti S, Severi S, Matteucci F, Zavoiu V, Mercatali L, Amadori D, Ibrahim T (2019) Ten-year experience of the multidisciplinary osteoncology center. Support Care Cancer 27(9):3395–3402. https://doi.org/10.1007/s00520-019-4635-5
van der Pol CB, Schweitzer ME, Di Primio G, Sampaio ML, Kielar A, Clemons M, Jaberi A (2014) Breast cancer and bone metastases: the association of axial skeleton MRI findings with skeletal-related events and survival. Breast Cancer Res Treat 146(3):583–589. https://doi.org/10.1007/s10549-014-3046-z
Jacobs C, Simos D, Addison C, Ibrahim M, Clemons M (2014) Pharmacotherapy of bone metastases in breast cancer patients–an update. Expert Opin Pharmacother 15(8):1109–1118. https://doi.org/10.1517/14656566.2014.903925
Kuchuk I, Hutton B, Moretto P, Ng T, Addison CL, Clemons M (2013) Incidence, consequences and treatment of bone metastases in breast cancer patients-experience from a single cancer centre. J Bone Oncol 2(4):137–144. https://doi.org/10.1016/j.jbo.2013.09.001
Cleeland CS, Body JJ, Stopeck A, von Moos R, Fallowfield L, Mathias SD, Patrick DL, Clemons M, Tonkin K, Masuda N, Lipton A, de Boer R, Salvagni S, Oliveira CT, Qian Y, Jiang Q, Dansey R, Braun A, Chung K (2013) Pain outcomes in patients with advanced breast cancer and bone metastases: results from a randomized, double-blind study of denosumab and zoledronic acid. Cancer 119(4):832–838. https://doi.org/10.1002/cncr.27789
Clemons M, Gelmon KA, Pritchard KI, Paterson AH (2012) Bone-targeted agents and skeletal-related events in breast cancer patients with bone metastases: the state of the art. Curr Oncol 19(5):259–268. https://doi.org/10.3747/co.19.1011
Holen I, Coleman RE (2010) Bisphosphonates as treatment of bone metastases. Curr Pharm Des 16(11):1262–1271. https://doi.org/10.2174/138161210791034003
Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, Apffelstaedt J, Hussein M, Coleman RE, Reitsma DJ, Seaman JJ, Chen BL, Ambros Y (2001) Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J 7(5):377–387
O'Carrigan B, Wong MH, Willson ML, Stockler MR, Pavlakis N, Goodwin A (2017) Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev 10:Cd003474. https://doi.org/10.1002/14651858.CD003474.pub4
Fernandes R, Siegel P, Komarova S, Hilton J, Addison C, Ibrahim MF, Werier J, Dennis K, Singh G, Amir E, Jarvis V, Emmenegger U, Mazzarello S, Clemons M (2016) Future directions for bone metastasis research - highlights from the 2015 bone and the Oncologist new updates conference (BONUS). J Bone Oncol 5(2):57–62. https://doi.org/10.1016/j.jbo.2016.02.004
Hutton B, Mazzarello S, Clemons M (2015) Dosing strategies of bone-targeting agents. JAMA Intern Med 175(11):1864–1865. https://doi.org/10.1001/jamainternmed.2015.4789
Zhu X, Amir E, Singh G, Clemons M, Addison C (2014) Bone-targeted therapy for metastatic breast cancer-where do we go from here? A commentary from the BONUS 8 meeting. J Bone Oncol 3(1):1–4. https://doi.org/10.1016/j.jbo.2014.01.001
Addison CL, Bouganim N, Hilton J, Vandermeer L, Dent S, Amir E, Hopkins S, Kuchuk I, Segal R, Song X, Gertler S, Mazzarello S, Dranitsaris G, Ooi D, Pond G, Clemons M (2014) A phase II, multicentre trial evaluating the efficacy of de-escalated bisphosphonate therapy in metastatic breast cancer patients at low-risk of skeletal-related events. Breast Cancer Res Treat 144(3):615–624. https://doi.org/10.1007/s10549-014-2906-x
Addison CL, Pond GR, Zhao H, Mazzarello S, Vandermeer L, Goldstein R, Amir E, Clemons M (2014) Effects of de-escalated bisphosphonate therapy on bone turnover biomarkers in breast cancer patients with bone metastases. Springerplus 3:577. https://doi.org/10.1186/2193-1801-3-577
Addison CL, Simos D, Wang Z, Pond G, Smith S, Robertson S, Mazzarello S, Singh G, Vandermeer L, Fernandes R, Iyengar A, Verma S, Clemons M (2016) A phase 2 trial exploring the clinical and correlative effects of combining doxycycline with bone-targeted therapy in patients with metastatic breast cancer. J Bone Oncol 5(4):173–179. https://doi.org/10.1016/j.jbo.2016.06.003
Kuchuk I, Beaumont JL, Clemons M, Amir E, Addison CL, Cella D (2013) Effects of de-escalated bisphosphonate therapy on the functional assessment of cancer therapy-bone pain, brief pain inventory and bone biomarkers. J Bone Oncol 2(4):154–157. https://doi.org/10.1016/j.jbo.2013.07.004
Amadori D, Aglietta M, Alessi B, Gianni L, Ibrahim T, Farina G, Gaion F, Bertoldo F, Santini D, Rondena R, Bogani P, Ripamonti CI (2013) Efficacy and safety of 12-weekly versus 4-weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): a phase 3, open-label, randomised, non-inferiority trial. Lancet Oncol 14(7):663–670. https://doi.org/10.1016/s1470-2045(13)70174-8
Amir E, Freedman O, Carlsson L, Dranitsaris G, Tomlinson G, Laupacis A, Tannock IF, Clemons M (2013) Randomized feasibility study of de-escalated (every 12 wk) versus standard (every 3 to 4 wk) intravenous pamidronate in women with low-risk bone metastases from breast cancer. Am J Clin Oncol 36(5):436–442. https://doi.org/10.1097/COC.0b013e3182568f7a
Lipton A, Steger GG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, de Boer R, Berardi R, Gascon P, Tonkin KS, Coleman R, Paterson AH, Peterson MC, Fan M, Kinsey A, Jun S (2007) Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol 25(28):4431–4437. https://doi.org/10.1200/jco.2007.11.8604
Ibrahim MF, Mazzarello S, Shorr R, Vandermeer L, Jacobs C, Hilton J, Hutton B, Clemons M (2015) Should de-escalation of bone-targeting agents be standard of care for patients with bone metastases from breast cancer? A systematic review and meta-analysis. Ann Oncol 26(11):2205–2213. https://doi.org/10.1093/annonc/mdv284
Awan AA, Hutton B, Hilton J, Mazzarello S, Van Poznak C, Vandermeer L, Bota B, Stober C, Sienkiewicz M, Fergusson D, Shorr R, Clemons M (2019) De-escalation of bone-modifying agents in patients with bone metastases from breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 176(3):507–517. https://doi.org/10.1007/s10549-019-05265-1
Hong BY, Ibrahim MF, Fernandes R, Mazzarello S, Hutton B, Shorr R, Clemons M (2016) De-escalation of bone-targeted agents for metastatic prostate cancer. Curr Oncol 23(1):e77-78. https://doi.org/10.3747/co.23.2913
Van Poznak C, Somerfield MR, Barlow WE, Biermann JS, Bosserman LD, Clemons MJ, Dhesy-Thind SK, Dillmon MS, Eisen A, Frank ES, Jagsi R, Jimenez R, Theriault RL, Vandenberg TA, Yee GC, Moy B (2017) Role of bone-modifying agents in metastatic breast cancer: an American Society of Clinical Oncology-Cancer Care Ontario focused guideline update. J Clin Oncol 35(35):3978–3986. https://doi.org/10.1200/jco.2017.75.4614
Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD, Giordano SH, Goetz MP, Goldstein LJ, Isakoff SJ, Krishnamurthy J, Lyons J, Marcom PK, Matro J, Mayer IA, Moran MS, Mortimer J, O’Regan RM, Patel SA, Pierce LJ, Rugo HS, Sitapati A, Smith KL, Smith ML, Soliman H, Stringer-Reasor EM, Telli ML, Ward JH, Young JS, Burns JL, Kumar R (2020) Breast cancer Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 18(4):452–478. https://doi.org/10.6004/jnccn.2020.0016
Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, Chow L, Sohn J, Lee KS, Campos-Gomez S, Villanueva-Vazquez R, Jung KH, Chakravartty A, Hughes G, Gounaris I, Rodriguez-Lorenc K, Taran T, Hurvitz S, Tripathy D (2019) Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med 381(4):307–316. https://doi.org/10.1056/NEJMoa1903765
James ND, Spears MR, Clarke NW, Dearnaley DP, De Bono JS, Gale J, Hetherington J, Hoskin PJ, Jones RJ, Laing R, Lester JF, McLaren D, Parker CC, Parmar MKB, Ritchie AWS, Russell JM, Strebel RT, Thalmann GN, Mason MD, Sydes MR (2015) Survival with newly diagnosed metastatic prostate cancer in the “docetaxel era”: data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019). Eur Urol 67(6):1028–1038. https://doi.org/10.1016/j.eururo.2014.09.032
Guarneri V, Donati S, Nicolini M, Giovannelli S, D’Amico R, Conte PF (2005) Renal safety and efficacy of iv bisphosphonates in patients with skeletal metastases treated for up to 10 years. Oncologist 10(10):842–848. https://doi.org/10.1634/theoncologist.10-10-842
Henk H, Teitelbaum A, Kaura S (2012) Evaluation of the clinical benefit of long-term (beyond 2 years) treatment of skeletal-related events in advanced cancers with zoledronic acid. Curr Med Res Opin 28(7):1119–1127. https://doi.org/10.1185/03007995.2012.689254
Clemons M, Liu M, Stober C, Pond G, Alzahrani MJ, Ong M, Ernst S, Booth C, Mates M, Joy AA (2021) Two-year results of a randomised trial comparing 4-versus 12-weekly bone-targeted agent use in patients with bone metastases from breast or castration-resistant prostate cancer. Journal of Bone Oncology:100388
Clemons M, Ong M, Stober C, Ernst S, Booth C, Canil C, Mates M, Robinson A, Blanchette P, Joy AA (2021) A randomised trial of 4-versus 12-weekly administration of bone-targeted agents in patients with bone metastases from breast or castration-resistant prostate cancer. Eur J Cancer 142:132–140
Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA, Chen B (2002) A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 94(19):1458–1468. https://doi.org/10.1093/jnci/94.19.1458
Major PP, Cook R (2002) Efficacy of bisphosphonates in the management of skeletal complications of bone metastases and selection of clinical endpoints. Am J Clin Oncol 25(6 Suppl 1):S10-18. https://doi.org/10.1097/00000421-200212001-00003
Ng TL, Tu MM, Ibrahim MFK, Basulaiman B, McGee SF, Srikanthan A, Fernandes R, Vandermeer L, Stober C, Sienkiewicz M, Jeong A, Saunders D, Awan AA, Hutton B, Clemons MJ (2020) Long-term impact of bone-modifying agents for the treatment of bone metastases: a systematic review. Support Care Cancer. https://doi.org/10.1007/s00520-020-05556-0
Coleman RE (2004) Bisphosphonates: clinical experience. Oncologist 9(Suppl 4):14–27. https://doi.org/10.1634/theoncologist.9-90004-14
Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA, Zheng M (2004) Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst 96(11):879–882. https://doi.org/10.1093/jnci/djh141
Liauw W, Segelov E, Lih A, Dunleavy R, Links M, Ward R (2005) Off-trial evaluation of bisphosphonates in patients with metastatic breast cancer. BMC Cancer 5:89. https://doi.org/10.1186/1471-2407-5-89
Hussain A, Aly A, Daniel Mullins C, Qian Y, Arellano J, Onukwugha E (2016) Risk of skeletal related events among elderly prostate cancer patients by site of metastasis at diagnosis. Cancer Med 5(11):3300–3309. https://doi.org/10.1002/cam4.914
Brufsky AM, Sereika SM, Mathew A, Tomifumi O, Singh V, Rosenzweig M (2013) Long-term treatment with intravenous bisphosphonates in metastatic breast cancer: a retrospective study. Breast J 19(5):504–511. https://doi.org/10.1111/tbj.12152
Lipton A, Theriault RL, Hortobagyi GN, Simeone J, Knight RD, Mellars K, Reitsma DJ, Heffernan M, Seaman JJ (2000) Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer 88(5):1082–1090. https://doi.org/10.1002/(sici)1097-0142(20000301)88:5%3c1082::aid-cncr20%3e3.0.co;2-z
Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, Apffelstaedt J, Hussein MA, Coleman RE, Reitsma DJ (2003) Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized double-blind multicenter comparative trial. Cancer: Interdisciplinary International Journal of the American Cancer Society 98(8):1735–1744
Hatoum HT, Lin SJ, Smith MR, Guo A, Lipton A (2011) Treatment persistence with monthly zoledronic acid is associated with lower risk and frequency of skeletal complications in patients with breast cancer and bone metastasis. Clin Breast Cancer 11(3):177–183. https://doi.org/10.1016/j.clbc.2011.03.015
Miyashita H, Cruz C, Malamud S (2020) Risk factors for skeletal-related events in patients with bone metastasis from breast cancer undergoing treatment with zoledronate. Breast Cancer Res Treat 182(2):381–388. https://doi.org/10.1007/s10549-020-05712-4
Templeton AJ, Stalder L, Sauvin LA, Bernhard J, Brauchli P, Gillessen S, Hayoz S, Klingbiel D, Matter-Walstra K, Thürlimann B (2014) Prevention of symptomatic skeletal events with denosumab administered every 4 weeks versus every 12 weeks–a non-inferiority phase III trial: Sakk 96/12-Reduse. Annals of Oncology 25:iv540
Acknowledgements
We are grateful to the Ottawa Hospital patients for their participation in the REaCT-BTA study.
Funding
The research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. This work was supported by the Rethinking Clinical Trials (REaCT) Program platform at the Ottawa Hospital which is supported by The Ottawa Hospital Foundation and its generous donors.
Author information
Authors and Affiliations
Contributions
MA, AA, CS, LV, and MC designed the study and prepared the protocol. MA and CS collected the data and coordinated the study, and GP did the statistical analysis. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. MA, GP, AA, CS, LV, ML, TN, and MC wrote the manuscript. All authors were involved in the critical review of the manuscript and approved the final version.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ontario Cancer Research Ethics Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All data has been de-identified to protect the identities of subjects involved in the research.
Consent to participate
Integrated verbal consent was obtained from all trial participants included in the study.
Consent for publication
All participants consented to de-identified publication of aggregate study results.
Conflict of interest
AA reports personal fees (honoraria) from Apotex, Eli Lily, Exact Sciences, Exactis, Novartis, Pfizer, and Roche outside the submitted work. TN reports personal fees (honoraria) from ARIAD, Takeda, and Boehringer-Ingelheim, outside the submitted work. GRP reports personal fees (honoraria) from Astra-Zeneca and Merck outside the submitted work, as well as a family member who works for Roche and owns stock in Roche. All other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alzahrani, M., Stober, C., Liu, M. et al. Symptomatic skeletal-related events in patients receiving longer term bone-modifying agents for bone metastases from breast and castration resistant prostate cancers. Support Care Cancer 30, 3977–3984 (2022). https://doi.org/10.1007/s00520-021-06714-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06714-8