Abstract
Purpose
Bone-modifying agents (BMAs) such as bisphosphonates and denosumab are usually administered every 4 weeks (standard) in patients with bone metastases from breast cancer to prevent skeletal-related events (SREs). Recent randomized controlled trials suggest every 12-week (de-escalated) dosing interval may be non-inferior. The objective of this systematic review and meta-analysis was to compare the efficacy and harms of standard with de-escalated administration of BMA’s in patients with bone metastases from breast cancer.
Methods
We searched Medline, PubMed, and the Cochrane Register of Controlled Trials from 1947 to March 14, 2018 and conference abstracts from (2014–March 14, 2018) for randomized clinical trials comparing every 4-week and every 12-week dosing interval of bone-modifying agents. Using PRISMA guidelines, meta-analyses were performed using random-effects models, with findings reported as risk ratios with 95% confidence intervals (CI).
Results
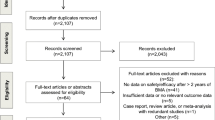
From a total of 1311 citations, we identified 8 full-text articles and 1 abstract comprising data from 5 completed randomized clinical trials (n = 1807). Zoledronate administration every 12 weeks compared to every 4 weeks produced a summary risk ratio of 1.05 (95% CI 0.88–1.25) for patients with ≥ 1 on-study SRE indicating similar efficacy. These results did not differ whether patients had received prior intravenous bisphosphonate. De-escalation was associated with a non-statistically significant lower risk of increased creatinine (summary risk ratio 0.41 [95% CI 0.15–1.16]). Currently, there are insufficient data for pamidronate and denosumab de-escalation.
Conclusions
These data are supportive of de-escalation of zoledronate from onset for patients with bone metastases from breast cancer.





Similar content being viewed by others
References
Coleman RE (1997) Skeletal complications of malignancy. Cancer-Am Cancer Soc 80(8 Suppl):1588–1594
Coleman RE, Rubens RD (1987) The clinical course of bone metastases from breast cancer. Br J Cancer 55(1):61–66
Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA (2009) Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat 115(2):423–428
Coleman RE (2001) Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27(3):165–176
Richards MA, Braysher S, Gregory WM, Rubens RD (1993) Advanced breast cancer: use of resources and cost implications. Br J Cancer 67(4):856–860
Body JJ, Chevalier P, Gunther O, Hechmati G, Lamotte M (2013) The economic burden associated with skeletal-related events in patients with bone metastases secondary to solid tumors in Belgium. J Med Econ 16(4):539–546
Clemons M, Gelmon KA, Pritchard KI, Paterson AHG (2012) Bone-targeted agents and skeletal-related events in breast cancer patients with bone metastases: the state of the art. Curr Oncol 19(5):259–268
Holen I, Coleman RE (2010) Bisphosphonates as treatment of bone metastases. Curr Pharm Des 16(11):1262–1271
Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, Apffelstaedt J, Hussein M, Coleman RE, Reitsma DJ et al (2001) Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J 7(5):377–387
Van Poznak C, Somerfield MR, Barlow WE, Biermann JS, Bosserman LD, Clemons MJ, Dhesy-Thind SK, Dillmon MS, Eisen A, Frank ES et al (2017) Role of bone-modifying agents in metastatic breast cancer: an American Society of Clinical Oncology-Cancer Care Ontario Focused Guideline Update. J Clin Oncol 35:3978
Van Poznak CH, Temin S, Yee GC, Janjan NA, Barlow WE, Biermann JS, Bosserman LD, Geoghegan C, Hillner BE, Theriault RL et al (2011) American Society of Clinical Oncology Executive Summary of the Clinical Practice Guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncol 29(9):1221–1227
Network NCC: Breast Cancer Version 1.2018 (April 23, 2018) (2018)
Hutton B, Addison C, Mazzarello S, Joy AA, Bouganim N, Fergusson D, Clemons M (2013) De-escalated administration of bone-targeted agents in patients with breast and prostate cancer—a survey of Canadian oncologists. J Bone Oncol 2(2):77–83
Van Poznak CH, Von Roenn JH, Temin S (2011) American society of clinical oncology clinical practice guideline update: recommendations on the role of bone-modifying agents in metastatic breast cancer. J Oncol Pract 7(2):117–121
Verma S, Kerr-Cresswell D, Dranitsaris G, Charbonneau F, Trudeau M, Yogendran G, Cesta AM, Clemons M (2004) Bisphosphonate use for the management of breast cancer patients with bone metastases: a survey of Canadian Medical Oncologists. Support Care Cancer 12(12):852–858
Amadori D, Aglietta M, Alessi B, Gianni L, Ibrahim T, Farina G, Gaion F, Bertoldo F, Santini D, Rondena R et al (2013) Efficacy and safety of 12-weekly versus 4-weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): a phase 3, open-label, randomised, non-inferiority trial. Lancet Oncol 14(7):663–670
Himelstein AL, Foster JC, Khatcheressian JL, Roberts JD, Seisler DK, Novotny PJ, Qin R, Go RS, Grubbs SS, O’Connor T et al (2017) Effect of longer-interval versus standard dosing of zoledronic acid on skeletal events in patients with bone metastases A randomized clinical trial. Jama-J Am Med Assoc 317(1):48–58
Hortobagyi GN, Van Poznak C, Harker WG, Gradishar WJ, Chew H, Dakhil SR, Haley BB, Sauter N, Mohanlal R, Zheng M et al (2017) Continued treatment effect of zoledronic acid dosing every 12 vs 4 weeks in women with breast cancer metastatic to bone: the OPTIMIZE-2 randomized clinical trial. Jama Oncol 3:906
Fernandes R, Siegel P, Komarova S, Hilton J, Addison C, Ibrahim MFK, Werier J, Dennis K, Singh G, Amir E et al (2016) Future directions for bone metastasis research—highlights from the 2015 bone and the Oncologist new updates conference (BONUS). J Bone Oncol 5(2):57–62
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA (2015) Grp P-P: preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Br Med J 2015:349
Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br Med J 343:d5928
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ, Grp GW (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Br Med J 336(7650):924–926
Schünemann H BJ, Guyatt G, Oxman A (eds) (2013) GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group, 2013
McMaster University dbEP, Inc. (2015) GRADEpro GDT: GRADEpro guideline development tool [Software]
Higgins JPT, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558
The Nordic Cochrane Centre TCC (2014) Copenhagen: review manager (RevMan) [Computer program]. Version 5.3
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj 339:b2535
Addison CL, Pond GR, Zhao HJ, Mazzarello S, Vandermeer L, Goldstein R, Amir E, Clemons M (2014) Effects of de-escalated bisphosphonate therapy on bone turnover biomarkers in breast cancer patients with bone metastases. Springerplus 3:577
Amir E, Freedman O, Carlsson L, Dranitsaris G, Tomlinson G, Laupacis A, Tannock IF, Clemons M (2013) Randomized feasibility study of de-escalated (every 12 week) versus standard (every 3 to 4 week) intravenous pamidronate in women with low-risk bone metastases from breast cancer. Am J Clin Oncol-Cancer 36(5):436–442
Coleman RE, Wright J, Houston S, Agrawal R, Purohit OPK, Hayward L, Simmonds P, Waterhouse A, Marshall H, Investigators B (2012) Randomized trial of marker-directed versus standard schedule zoledronic acid for bone metastases from breast cancer. J Clin Oncol 30(15):511
Kuchuk I, Beaumont JL, Clemons M, Amir E, Addison CL, Cella D (2013) Effects of de-escalated bisphosphonate therapy on the functional assessment of cancer therapy-bone pain, brief pain inventory and bone biomarkers. J Bone Oncol 2(4):154–157
Lipton A, Steger GG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, de Boer R, Berardi R, Gascon P, Tonkin KS et al (2007) Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol 25(28):4431–4437
Lipton A, Steger GG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, de Boer R, Berardi R, Gascon P, Tonkin KS et al (2008) Extended efficacy and safety of denosumab in breast cancer patients with bone metastases not receiving prior bisphosphonate therapy. Clin Cancer Res 14(20):6690–6696
Templeton AJ, Stalder L, Bernhard J, Brauchli P, Gillessen S, Hayoz S, KlIngblel D, Matter-Walstra K, Thurlimann BJK, Von Moos R (2014) Prevention of symptomatic skeletal events with denosumab administered every 4 weeks versus every 12 weeks: a noninferiority phase III trial (SA 96/12, REDUSE). J Clin Oncol 32(15):20
Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, Gao G, Wu L, Sohn W, Jun S (2009) Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol 27(10):1564–1571
National Cancer Institute PROCSG (2006) Common Terminology Criteria for Adverse Events (CTCAE) v3.0
Smith MR, Coleman RE, Klotz L, Pittman K, Milecki P, Ng S, Chi KN, Balakumaran A, Wei R, Wang H et al (2015) Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann Oncol 26(2):368–374
Ibrahim MFK, Clemons MJ, Hutton B, Hilton JF, Vandermeer L, Mazzarello S, Shorr R (2015) Should de-escalation of bone-targeted agents be standard of care for patients with bone metastases from breast cancer? A systematic review and meta-analysis. J Clin Oncol 26:2205
Cao L, Yang YJ, Diao JD, Zhang XH, Liu YL, Wang BY, Li ZW, Liu SX (2017) Systematic review and meta-analysis comparing zoledronic acid administered at 12-week and 4-week intervals in patients with bone metastasis. Oncotarget 8(52):90308–90314
Yanae M, Fujimoto S, Tane K, Tanioka M, Fujiwara K, Tsubaki M, Yamazoe Y, Morishima Y, Chiba Y, Takao S et al (2017) Increased risk of SSEs in bone-only metastatic breast cancer patients treated with zoledronic acid. J Bone Oncol 8:18–22
Shapiro CL, Moriarty JP, Dusetzina S, Himelstein AL, Foster JC, Grubbs SS, Novotny PJ, Borah BJ (2017) Cost-effectiveness analysis of monthly zoledronic acid, zoledronic acid every 3 months, and monthly denosumab in women with breast cancer and skeletal metastases: CALGB 70604 (alliance). J Clin Oncol 35:3949
Clemons M (2016) A pragmatic randomised, multicentre trial comparing 4-weekly versus 12-weekly administration of bone-targeted agents in patients with bone metastases from either castration-resistant prostate cancer or breast cancer—the REaCT-BTA Study. Clinicaltrialsgov NCT02721433
Funding
This project received no outside funding and was supported with internal research funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Awan reports participating in the Novartis Canada Advisory Board on the use of Ribociclib. Dr. Hutton reports personal fees from Cornerstone Research, outside the submitted work. Dr. van Poznak reports personal fees from UpToDate and institutional research funds from Bayer, outside the submitted work. The remaining authors declare that they have no conflicts of interest (Hilton, Mazzarello, Vandermeer, Bota, Stober, Sienkiewicz, Fergusson, Shorr, and Clemons).
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Not applicable as this is a systematic review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Awan, A.A., Hutton, B., Hilton, J. et al. De-escalation of bone-modifying agents in patients with bone metastases from breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 176, 507–517 (2019). https://doi.org/10.1007/s10549-019-05265-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05265-1