Abstract
Purpose
CINV remains a distressing side effect experienced by glioma patients receiving multi-day temozolomide therapy, in spite of guideline-based antiemetic therapy with selective serotonin-receptor-antagonists. Antiemetic research with aprepitant has routinely excluded glioma patients. In this randomized open-label phase II study, use of a nonstandard 5-day regimen of aprepitant for glioma patients was investigated.
Methods
One hundred thirty-six glioma patients receiving their first cycle of adjuvant temozolomide (150–200 mg/m2/day × 5 days every 28 days) were randomized to Arm-A (ondansetron 8 mg days 1–5 with aprepitant day 1: 125 mg, days 2–5: 80 mg) or Arm-B (ondansetron). Randomization was stratified by tumor grade and number of prior chemotherapy regimens. The primary endpoint was the percentage of patients achieving complete control (CC), defined as no emetic episode or antiemetic rescue medication over the 7-day study period. Secondary endpoints included CINV efficacy in the acute phase (≤ 24 h) and delayed phase (days 2–7), as well as safety and quality of life (QoL).
Results
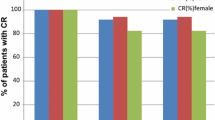
Patients were 61% male, 97% white, 48% with KPS > 90%, 60% non-smokers, mean age 54, 92% with low alcohol use, and 46% with a CINV history. The CC was 58.6% (Arm-A) and 54.5% (Arm-B). Acute-complete response (CR) rates, defined as CC on day 1 in Arm-A and -B, were 97.1% and 87.9%, respectively (p = 0.056). Treatment-related toxicities were mild or moderate in severity.
Conclusions
Aprepitant plus ondansetron may increase acute-CR, may have benefit regarding CINV’s effect on QoL, and is safe for 5-day temozolomide compared to ondansetron. This study provides no evidence that aprepitant increases CC rate over ondansetron alone.




Similar content being viewed by others
References
Abdel-Rahman O (2016) Neurokinin-1 inhibitors in the prevention of nausea and vomiting from highly emetogenic chemotherapy: a network meta-analysis. Ther Adv Med Oncol 8(5):396–406. https://doi.org/10.1177/1758834016654902
Aapro M (2018) Searching for perfection: further progress in management of chemotherapy-induced nausea and vomiting-concluding thoughts. Support Care Cancer 26(Suppl 1):35–37. https://doi.org/10.1007/s00520-018-4121-5
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996. https://doi.org/10.1056/NEJMoa043330
Affronti ML, Woodring S, Peters KB, Herndon JE 2nd, McSherry F, Healy PN, Desjardins A, Vredenburgh JJ, Friedman HS (2017) A phase II single-arm trial of palonosetron for the prevention of acute and delayed chemotherapy-induced nausea and vomiting in malignant glioma patients receiving multidose irinotecan in combination with bevacizumab. Ther Clin Risk Manag 13:33–40. https://doi.org/10.2147/TCRM.S122480
Affronti ML, Schneider SM, Herndon JE 2nd, Schlundt S, Friedman HS (2014) Adherence to antiemetic guidelines in patients with malignant glioma: a quality improvement project to translate evidence into practice. Support Care Cancer 22(7):1897–1905. https://doi.org/10.1007/s00520-014-2136-0
Affronti ML, Woodring S, Allen K, Kirkpatrick J, Peters KB, Herndon JE 2nd, McSherry F, Healy PN, Desjardins A, Vredenburgh JJ, Friedman HS (2016) Phase II study to evaluate the safety and efficacy of intravenous palonosetron (PAL) in primary malignant glioma (MG) patients receiving standard radiotherapy (RT) and concomitant temozolomide (TMZ). Support Care Cancer 24(10):4365–4375. https://doi.org/10.1007/s00520-016-3276-1
(NCCN) (2011, 2012) National comprehensive cancers network: clinical practice guidelines in oncology. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed 2012
Roila F, Molassiotis A, Herrstedt J, Aapro M, Gralla RJ, Bruera E, Clark-Snow RA, Dupuis LL, Einhorn LH, Feyer P, Hesketh PJ, Jordan K, Olver I, Rapoport BL, Roscoe J, Ruhlmann CH, Walsh D, Warr D, van der Wetering M (2016) Participants of the MECCC (2016) 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 27(suppl 5):v119–v133. https://doi.org/10.1093/annonc/mdw270
Hesketh PJ, Bohlke K, Kris MG (2017) Antiemetics: American Society of Clinical Oncology clinical practice guideline update summary. J Oncol Pract 13(12):825–830. https://doi.org/10.1200/JOP.2017.026351
Rozzi A, Nardoni C, Corona M, Restuccia MR, Fabi A, Bria E, Minniti G, Lanzetta G (2011) Palonosetron for the prevention of chemotherapy-induced nausea and vomiting in glioblastoma patients treated with temozolomide: a phase II study. Support Care Cancer 19(5):697–701. https://doi.org/10.1007/s00520-010-0893-y
Molassiotis A, Coventry PA, Stricker CT, Clements C, Eaby B, Velders L, Rittenberg C, Gralla RJ (2007) Validation and psychometric assessment of a short clinical scale to measure chemotherapy-induced nausea and vomiting: the MASCC antiemesis tool. J Pain Symptom Manag 34(2):148–159. https://doi.org/10.1016/j.jpainsymman.2006.10.018
Martin CG, Rubenstein EB, Elting LS, Kim YJ, Osoba D (2003) Measuring chemotherapy-induced nausea and emesis. Cancer 98(3):645–655. https://doi.org/10.1002/cncr.11540
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) European organisation for R, treatment of cancer brain T, radiotherapy G, National Cancer Institute of Canada clinical trials G (2005) radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996. https://doi.org/10.1056/NEJMoa043330
Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, Danso MA, Dennis K, Dupuis LL, Dusetzina SB, Eng C, Feyer PC, Jordan K, Noonan K, Sparacio D, Somerfield MR, Lyman GH (2017) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 35(28):3240–3261. https://doi.org/10.1200/JCO.2017.74.4789
Yuan DM, Li Q, Zhang Q, Xiao XW, Yao YW, Zhang Y, Lv YL, Liu HB, Lv TF, Song Y (2016) Efficacy and safety of Neurokinin-1 receptor antagonists for prevention of chemotherapy-induced nausea and vomiting: systematic review and meta-analysis of randomized controlled trials. Asian Pac J Cancer Prev 17(4):1661–1675
Clemons M (2018) Guidelines versus individualized care for the management of CINV. Support Care Cancer 26(Suppl 1):11–17. https://doi.org/10.1007/s00520-018-4115-3
Aapro M (2018) CINV: still troubling patients after all these years. Support Care Cancer 26(Suppl 1):5–9. https://doi.org/10.1007/s00520-018-4131-3
Malhotra M, Chandrasekahran A, Tonse R, Jalali R, Patil VM (2019) Cross-sectional study of temozolomide-induced chemotherapy-induced nausea and vomiting in patients with glioma. Clin Oncol (R Coll Radiol) 31(1):e85. https://doi.org/10.1016/j.clon.2018.10.008
Matsuda M, Yamamoto T, Ishikawa E, Nakai K, Akutsu H, Onuma K, Matsumura A (2015) Profile analysis of chemotherapy-induced nausea and vomiting in patients treated with concomitant Temozolomide and radiotherapy: results of a prospective study. Neurol Med Chir (Tokyo) 55(9):749–755. https://doi.org/10.2176/nmc.oa.2014-0413
Affronti M, Randazzo D, McSherry F, Healy P, Herndon II J, Weant M, Miller E, Lipp E, Friedman H, Peters K (2018) Review and meta-analysis of nausea and vomiting trials for malignant gliomas. In: 23rd annual scientific meeting and education day of the Society for Neuro-Oncology, New Orleans, LA. vol 6. Oxford University Press, p vi218
Patil VM, Chandrasekharan A, Vallathol DH, Malhotra M, Abhinav R, Agarwal P, Rajpurohit A, Tonse R, Bhattacharjee A, Jalali R (2019) Antiemetic prophylaxis with temozolomide: an audit from a tertiary care center. Neurooncol Pract. https://doi.org/10.1093/nop/npz009
Affronti ML, Bubalo J (2014) Palonosetron in the management of chemotherapy-induced nausea and vomiting in patients receiving multiple-day chemotherapy. Cancer Manag Res 6:329–337. https://doi.org/10.2147/cmar.s68102
Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ Jr, Mehta MP (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370(8):699–708. https://doi.org/10.1056/NEJMoa1308573
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370(8):709–722. https://doi.org/10.1056/NEJMoa1308345
Kang HJ, Loftus S, Taylor A, DiCristina C, Green S, Zwaan CM (2015) Aprepitant for the prevention of chemotherapy-induced nausea and vomiting in children: a randomised, double-blind, phase 3 trial. Lancet Oncol 16(4):385–394. https://doi.org/10.1016/S1470-2045(15)70061-6
Ng TL, Hutton B, Clemons M (2015) Chemotherapy-induced nausea and vomiting: time for more emphasis on nausea? Oncologist 20(6):576–583. https://doi.org/10.1634/theoncologist.2014-0438
Hernandez Torres C, Mazzarello S, Ng T, Dranitsaris G, Hutton B, Smith S, Munro A, Jacobs C, Clemons M (2015) Defining optimal control of chemotherapy-induced nausea and vomiting-based on patients' experience. Support Care Cancer 23(11):3341–3359. https://doi.org/10.1007/s00520-015-2801-y
Di Maio M, Gallo C, Leighl NB, Piccirillo MC, Daniele G, Nuzzo F, Gridelli C, Gebbia V, Ciardiello F, De Placido S, Ceribelli A, Favaretto AG, de Matteis A, Feld R, Butts C, Bryce J, Signoriello S, Morabito A, Rocco G, Perrone F (2015) Symptomatic toxicities experienced during anticancer treatment: agreement between patient and physician reporting in three randomized trials. J Clin Oncol 33(8):910–915. https://doi.org/10.1200/JCO.2014.57.9334
Salsman JM, Grunberg SM, Beaumont JL, Rogers M, Paul D, Clayman ML, Cella D (2012) Communicating about chemotherapy-induced nausea and vomiting: a comparison of patient and provider perspectives. J Natl Compr Cancer Netw 10(2):149–157
Grunberg SM, Deuson RR, Mavros P, Geling O, Hansen M, Cruciani G, Daniele B, De Pouvourville G, Rubenstein EB, Daugaard G (2004) Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer 100(10):2261–2268. https://doi.org/10.1002/cncr.20230
Acknowledgements
We would like to acknowledge Wendy R. Gentry for her formatting and development of tables and figures.
Funding
Merck provided aprepitant and funding for this investigator-initiated study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and informed consent
All study processes involving human subjects were performed in accordance with the ethical standards of the institutional review board of the Duke University Medical Center. All study participants voluntarily provided written informed consent prior to study inclusion.
Conflict of interest
Mary Lou Affronti has been supported by Eisai and Merck for investigator initiated clinical trials, served as an antiemetic expert advisory board member with Eisai for NEPA (2013–2014) and for Merck on an Advisory Expert Input forum (2017). Sarah Woodring, Annick Desjardins, Eric Lipp, and Elizabeth Miller received support from grant by Merck with their involvement during the conduct of the study. All other authors declare no conflict of interest at this time.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 135 kb)
Rights and permissions
About this article
Cite this article
Patel, M.P., Woodring, S., Randazzo, D.M. et al. Randomized open-label phase II trial of 5-day aprepitant plus ondansetron compared to ondansetron alone in the prevention of chemotherapy-induced nausea-vomiting (CINV) in glioma patients receiving adjuvant temozolomide. Support Care Cancer 28, 2229–2238 (2020). https://doi.org/10.1007/s00520-019-05039-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-05039-x