Abstract
Numerous groups have published guidelines for the prevention and management of chemotherapy-induced nausea and vomiting (CINV). The current management of CINV, however, remains suboptimal, due in part to poor adherence to existing antiemetic guidelines. Challenges in clinical trial design have also slowed progress and complicated the selection of optimal antiemetic therapy. In addition, patient-specific characteristics and factors are not included in current CINV guidelines and are an important contributor to an individual’s risk for nausea and vomiting. CINV risk prediction algorithms have now emerged and provide the opportunity to individualize antiemetic prophylaxis. Further studies are underway to examine the precise role for risk model-guided antiemetic prophylaxis in patients with cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the existence of numerous antiemetic therapies with demonstrated efficacy in preventing chemotherapy-induced nausea and vomiting (CINV), the management of CINV remains suboptimal. This disconnection is in part related to failure to follow guideline-directed therapy from groups such as the Multinational Association of Supportive Care in Cancer/European Society for Medical Oncology (MASCC/ESMO), the American Society of Clinical Oncology (ASCO), and the National Comprehensive Cancer Network (NCCN) [1,2,3]. Unfortunately however, even when following these guidelines, CINV and the control of nausea, in particular, for many patients remain poor. If we are to further reduce the incidence of CINV, we will need to develop strategies that not only lead to the availability of more effective antiemetic regimens, but we will also need to individualize patient care based on their personal risk of CINV.
Summary of current antiemetic guidelines
Chemotherapy-induced nausea and vomiting guidelines are primarily based on the emetogenic potential of chemotherapeutic agents when administered without any antiemetic prophylaxis. There are four broad categories: highly emetogenic chemotherapy (HEC; > 90% risk of emesis), moderately emetogenic chemotherapy (MEC; < 30 to 90% risk), low emetogenic chemotherapy (10 to 30% risk), and minimal emetogenic chemotherapy (< 10% risk) [1,2,3,4]. Table 1 shows the emetogenic classification of common anticancer therapies according to current consensus guidelines [1,2,3].
Currently available consensus guidelines demonstrate broad agreement on the key principles of CINV prophylaxis [1,2,3]. Prophylaxis should be initiated prior to chemotherapy for any patient with a 10% or greater risk of emesis and should continue for long enough to cover the duration of emetic risk. Antiemetic therapy should be based on the chemotherapeutic agents administered; for combination chemotherapy regimens, the agent with the highest emetogenic potential should guide selection of antiemetic therapy. Current CINV guidelines are summarized in Table 2 [1,2,3].
All three consensus guidelines agree that patients receiving HEC, including anthracycline/cyclophosphamide (AC)-based chemotherapy, should be treated with at least a three-drug antiemetic regimen consisting of a neurokinin-1 (NK-1) receptor antagonist, a 5-hydroxytryptamine (5-HT3) receptor antagonist, and dexamethasone [1,2,3]. The 2017 updated ASCO guidelines recommend the inclusion of olanzapine for all patients receiving HEC or AC combinations [3]. The MASCC/ESMO and NCCN guidelines also include the addition of olanzapine as an option for patients receiving HEC [1, 2]. If an NK-1 receptor antagonist is not available for prophylaxis in a patient receiving AC, the MASCC/ESMO guidelines recommend palonosetron as the preferred 5-HT3 receptor antagonist [1, 5], while the other two guidelines do not specify a preferred agent [2, 3].
For patients receiving MEC, NCCN guidelines recommend a 5-HT3 receptor antagonist and dexamethasone with or without either an NK-1 receptor antagonist or olanzapine [2]. The MASCC/ESMO guidelines and updated ASCO guidelines do not recommend incorporation of an NK-1 receptor antagonist for MEC unless carboplatin is used (discussed below) [1, 3]. In all three guidelines, the use of dexamethasone after day 1 is optional and should be considered if the MEC has a known potential for delayed CINV [1,2,3].
General principles for breakthrough and refractory CINV include the addition of another antiemetic agent with a different mechanism of action [2, 3]. For example, adding in an NK-1 receptor antagonist, olanzapine, benzodiazepine, cannabinoid, or dopamine receptor antagonist, or changing the 5-HT3 receptor antagonist.
Important changes in current CINV guidelines
There have been several significant updates to the consensus guidelines for CINV prophylaxis [1,2,3]. In addition to the many novel agents now added to the emetogenic classification categories (over 40 new drugs added in the recently updated MASCC/ESMO CINV guidelines), AC has been reclassified as HEC instead of MEC [1,2,3]. Chemotherapy-induced nausea and vomiting prophylaxis for carboplatin has also been revised in the MASCC/ESMO, NCCN, and ASCO guidelines. Although carboplatin is classified as MEC [1,2,3], the emetogenic potential is at the higher end of the MEC range (82 to 84% according to clinical trials) [5]. Recent phase II and phase III studies have shown that adding an NK-1 receptor antagonist to a 5-HT3 receptor antagonist and dexamethasone therapy can increase the complete response (CR; no vomiting and no use of rescue medication) by approximately 10 to 15% in patients receiving carboplatin chemotherapy [1, 6, 7]. As a result, current MASCC/ESMO guidelines recommend incorporation of an NK-1 receptor antagonist such as aprepitant, fosaprepitant, netupitant, or rolapitant [1]. NCCN and ASCO guidelines also recommend inclusion of an NK-1 receptor antagonist for patients receiving carboplatin at does resulting in an area under the curve (AUC) ≥ 4 mg/mL per minute [2, 3].
Olanzapine is now included in the NCCN guidelines for treatment of breakthrough CINV and as an alternative to an NK-1 receptor antagonist in combination with palonosetron and dexamethasone for prevention of CINV in patients receiving HEC or MEC [2]. In addition, olanzapine can be added as a fourth agent with aprepitant or fosaprepitant, a 5-HT3 receptor antagonist, and dexamethasone for patients receiving HEC. Prophylactic olanzapine is administered at a dose of 10 mg orally on days 1 through 4 for patients receiving HEC and on days 1 through 3 for those receiving MEC. However, the guidelines include a recommendation to consider a lower dose of 5 mg in elderly patients or those who experience sedation with 10 mg of olanzapine. Recent ASCO guidelines also suggest olanzapine should be administered as a four-drug regimen in combination with a 5-HT3 receptor antagonist, an NK-1 receptor antagonist, and dexamethasone in all patients receiving HEC, including those receiving an AC-based combination [3]. Olanzapine is also recommended for patients experiencing breakthrough nausea and vomiting in the ASCO guidelines. In the most recent MASCC/ESMO guidelines, olanzapine is listed as an option with a 5-HT3 receptor antagonist and dexamethasone for prophylaxis of CINV, particularly when nausea is an issue [1]. An extended-release formulation of granisetron for subcutaneous injection was also added to the NCCN guidelines as a reasonable 5-HT3 receptor antagonist option for combination antiemetic therapy [2].
Dexamethasone-sparing strategies have also been included in the NCCN guidelines for patients without significant CINV risk factors who are receiving MEC or non-cisplatin HEC based on data from several studies showing no significant increase in CINV when dexamethasone was only administered on day 1 [2, 8,9,10].
Optimizing antiemetic therapy: the challenges in clinical trial design
Despite the availability of multiple guidelines, many patients still have poorly controlled CINV [11,12,13,14]. The nature of clinical trials in this field presents considerable challenges and contributes to the difficulty in selecting optimal therapy. For instance, there is considerable diversity in the clinical trial endpoints reported for each study, making cross-trial comparisons difficult, especially as most trials simply ignore measures of nausea in their primary endpoints [15]. In a meta-analysis of 30 randomized clinical trials in CINV, comprehensive outcome measures such as total control (no vomiting, no nausea, and no use of rescue medications) and complete protection (no vomiting, no significant nausea, and no use of rescue medications) were reported in less than 25% of the trials. Clinical trials commonly focus on vomiting and underreport outcomes related to nausea control. Nausea is a particularly important outcome measure given that it occurs more frequently than vomiting and significantly decreases patient quality of life [16]. Unfortunately, many clinical trials use CR (no vomiting and no use of rescue medications) as their primary endpoint, which may not accurately reflect the patients’ actual experience with CINV.
Another issue is the small patient numbers in many of the CINV trials and a lack of head-to-head randomized clinical trial data to definitively establish which antiemetic agents are most effective against CINV. Lastly, several clinical trials investigating antiemetic therapies have been criticized for utilizing a suboptimal control arm not consistent with standard practice patterns. In order to improve our understanding of the CINV landscape and effectively evaluate the available data, there is a need for future clinical trials to use consistent reporting of outcomes based on a uniform definition. Nausea should be included as a part of the primary study outcome to better gauge the effectiveness of CINV control and patients’ experience. Guideline-based antiemetic therapy should also be used as the control arm for CINV clinical trials to improve the integrity of the results. In addition, all emesis data should be publicly and freely available so that investigators can compare the effects of different antiemetic regimens.
Beyond the guidelines: individualizing CINV prophylaxis
The field of oncology is moving toward a more precise, individualized approach that incorporates biomarkers and patient-related characteristics into treatment decision-making. Unfortunately, the recommendations of national and international consensus guidelines are based largely on the emetogenic potential of chemotherapeutic agents when given in the absence of any antiemetic therapy and show less consideration for other therapy-related and patient-related risk factors.
Beyond the emetogenic potential of the chemotherapeutic agents, therapy-related factors that contribute to CINV include drug dosage, treatment schedule, route of administration, and combinations with other emetogenic agents or radiation therapy [17, 18]. Established patient-related risk factors include young age (< 55 years), female gender, history of low alcohol intake, motion sickness, and prior emesis during pregnancy [19,20,21]. In addition, patient anxiety, expectation of emesis, metabolic abnormalities, gastrointestinal irritation, and intracranial pressure can also contribute to CINV [17, 19, 22,23,24]. Risk factors for anticipatory CINV have also been assessed and include CINV in a previous cycle of therapy, metastatic disease, and higher levels of anxiety prior to chemotherapy administration [25]. Ongoing studies continue to explore additional potential risk factors.
A personalized approach to CINV that incorporates these patient-related risk factors into antiemetic recommendations could significantly improve the management of CINV. For example, the range of emetogenic risk for MEC is extremely wide (30 to 90% risk of emesis), making it challenging for current CINV consensus guidelines to provide appropriate recommendations for every clinical scenario [2]. Patients receiving MEC with additional personal risk factors may benefit from the addition of other antiemetics. On the other hand, the majority of patients who are at lower risk of CINV can be safely treated without additional agents, reducing the risks of drug side effects and the financial toxicity of these agents.
Several CINV risk prediction models have been developed, including two repeated measures cycle-based models that predict the risk for acute and delayed CINV [26, 27]. A repeated measure approach allows CINV risk to be continually reassessed prior to each cycle of chemotherapy, rather than relying only on a single evaluation of baseline data to inform CINV prophylaxis for the entire course of cancer treatment. Predictors of CINV in these models included younger age, platinum or anthracycline-based chemotherapy, history of motion sickness or morning sickness, low daily alcohol consumption, patients’ expectation of nausea, and emesis in previous cycles of chemotherapy. Both CINV risk models demonstrated good predictive accuracy, with high-risk patients three times more likely to develop acute or delayed CINV than low-risk patients [28].
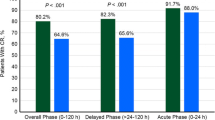
These models were subsequently evaluated in a clinical trial in which patients with breast cancer receiving anthracycline- and cyclophosphamide-containing chemotherapy regimens were randomized to either risk model-guided antiemetic prophylaxis or physician’s choice prophylaxis [26]. In the risk model-guided arm, low-risk patients received a 5-HT3 antagonist and dexamethasone, while high-risk patients also received aprepitant with or without olanzapine based on their risk of CINV. Risk model-guided prophylaxis was more effective than prophylaxis according to the treating physician’s discretion in preventing acute nausea and vomiting (53.7% with no nausea vs 41.6%; P < .001 and 91.8% with no vomiting vs 82.2%; P < .001) and delayed nausea and vomiting (39.6% with no nausea vs 30.7%; P = .01 and 87.1% with no vomiting vs 78.0%; P < .001).
These models are somewhat limited by the small sample size and geographic region used for their development, leading to a multinational collaborative effort to develop a new repeated measures prediction model based on a larger data set of almost 1200 patients from 5 prospective CINV studies [29]. This model was designed to assess individualized risk of grade ≥ 2 CINV (≥ 2 vomiting episodes or a decrease in oral intake due to nausea) over the first 5 days from chemotherapy administration. Eight risk factors were significantly associated with CINV risk: age < 60 years, anticipatory nausea and vomiting, sleep < 7 h the night before chemotherapy, history of morning sickness, use of non-prescribed antiemetics (e.g., dimenhydrinate, antacids, herbal supplements), receiving platinum or anthracycline-based chemotherapy, first-line of chemotherapy, and the occurrence of CINV in the previous chemotherapy cycle. These factors were combined into a risk prediction model that showed good predictive accuracy with an area under the curve of 0.69 (95% confidence interval 0.67–0.70) (Fig. 1) [29]. On a 32-point scale, the threshold for high risk was defined as a score of ≥ 16, which correlates with a CINV risk of at least 60%. However, the authors add that this threshold is not fixed and may be adjusted to reflect the risk tolerance of patients and clinicians. This risk model has now been made freely available online at http://www.cinvrisk.org for patients and oncologists to use.
Risk scoring algorithm for grade ≥ 2 CINV [29]. aThe probability of developing ≥ grade 2 CINV during that cycle of therapy can then be estimated from the accompanying graph
The development of risk prediction algorithms with high sensitivity and specificity will likely aid decision-making and improve CINV prophylaxis in the future. Risk model-guided antiemetic prophylaxis may also be more economical than physician choice prophylaxis. A recent cost-utility analysis of these two approaches showed that risk model-guided therapy was cost effective and was associated with gains in quality-adjusted life years [30]. However, this area of CINV management is a work in progress and further randomized studies are needed to fully elucidate the role of these prediction models in individualization of patient care. For example, my group is currently leading a large randomized phase III trial in which patients with newly diagnosed breast cancer receiving anthracycline/cyclophosphamide-containing regimens or platinum-based chemotherapy regimens are randomized to either doublet (5-HT3 receptor antagonist and dexamethasone) or triplet (NK-1 and 5-HT3 receptor antagonists plus dexamethasone) antiemetic therapy based on their individual risk [31]. This study is also evaluating the role of olanzapine at a dose of 5 mg in these patients, as we know many patients will still have significant nausea even when receiving “optimal” guideline-recommended antiemetic therapy. The need for more personalized antiemetic approaches is reflected in the current NCCN guidelines, which state that decisions regarding CINV prophylaxis should be “individualized for each chemotherapy regimen and each patient” [2]. It is our hope that the introduction of “individualized” antiemetic therapy will lead to a move away from the current cookie cutter recommendations of guideline groups, as ultimately personalized therapy will improve CINV control and enhance the quality of life of our patients.
References
Roila F, Molassiotis A, Herrstedt J, participants of the MASCC/ESMO Consensus Conference Copenhagen 2015 et al (2016) 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 27(suppl 5):v119–v133 http://www.mascc.org/assets/Guidelines-Tools/mascc_antiemetic_guidelines_english_2016_v.1.2.pdf. Accessed 1 July 2017
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Antiemesis. Version 2.2017. Available at: https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed 1 July 2017.
Hesketh PJ, Kris MG, Basch E et al (2017) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 35:3240–3261
Grunberg SM, Osoba D, Hesketh PJ et al (2005) Evaluation of new antiemetic agents and definition of antineoplastic agent emetogenicity—an update. Support Cancer Cancer 13:80–84
Hannigan EV, Green S, Alberts DS, O’Toole R, Surwit E (1993) Results of a Southwest Oncology Group phase III trial of carboplatin plus cyclophosphamide versus cisplatin plus cyclophosphamide in advanced ovarian cancer. Oncology 50(Suppl 2):2–9
Ito Y, Karayama M, Inui N et al (2014) Aprepitant in patients with advanced non-small-cell lung cancer receiving carboplatin-based chemotherapy. Lung Cancer 84:259–264
Hesketh PJ, Schnadig ID, Schwartzberg LS et al (2016) Efficacy of the neurokinin-1 receptor antagonist rolapitant in preventing nausea and vomiting in patients receiving carboplatin-based chemotherapy. Cancer 122:2418–2425
Aapro M, Fabi A, Nole F et al (2010) Double-blind, randomised, controlled study of the efficacy and tolerability of palonosetron plus dexamethasone for 1 day with or without dexamethasone on days 2 and 3 in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy. Ann Oncol 21:1083–1088
Matsuzaki K, Ito Y, Fukuda M et al (2016) Placebo-controlled phase III study comparing dexamethasone on day 1 to on day 1–3 with NK1 receptor antagonist and palonosetron in high emetogenic chemotherapy. J Clin Oncol 34(suppl): Abstract 10019
Roila F, Ruggeri B, Ballatori E, Del Favero A, Tonato M (2014) Aprepitant versus dexamethasone for preventing chemotherapy-induced delayed emesis in patients with breast cancer: a randomized double-blind study. J Clin Oncol 32:101–106
Beusterin K, Grinspan J, Kuchuk I et al (2014) Use of conjoint analysis to assess breast cancer patient preferences for chemotherapy side effects. Oncologist 19:127–134
Kuchuk I, Bouganim N, Beusterien N et al (2013) Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res Treat 142:101–107
Laszlo J, Lucas VS Jr (1981) Emesis as a critical problem in chemotherapy. N Engl J Med 305:948–949
Teunissen AD, Wesker W, Kruitwagen C, de Haes HC, Voest EE, deGraeff A (2007) Symptom prevalence in patients with incurable cancer: A symptomatic review. J Pain Symptom Manag 34:94–104
Ng T, Mazzarello S, Wang Z et al (2016) Choice of study endpoint significantly impacts the results of breast cancer trials evaluating chemotherapy-induced nausea and vomiting. Breast Cancer Res Treat 155:337–344
Ng TL, Hutton B, Clemons M (2015) Chemotherapy-induced nausea and vomiting: time for more emphasis on nausea? Oncologist 20:576–583
Bayo J, Fonseca PJ, Hernando S et al (2012) Chemotherapy-induced nausea and vomiting: pathophysiology and therapeutic principles. Clin Transl Oncol 14:413–422
Fraunholz I, Grau K, Weiss C, Rödel C (2011) Patient- and treatment-related risk factors for nausea and emesis during concurrent chemoradiotherapy. Strahlenther Oncol 187:1–6
Warr D (2014) Prognostic factors for chemotherapy induced nausea and vomiting. Eur J Pharmacol 722:192–196
Hesketh PK, Aapro M, Street JC, Carides AD (2010) Evaluation of risk factors predictive of nausea and vomiting with current state-of-the-art antiemetic treatment: analysis of two phase III trials of aprepitant in patients receiving cisplatin-based chemotherapy. Support Care Cancer 18:1171–1177
Warr DG, Street JC, Carides AD (2011) Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: analysis of phase 3 trial of aprepitant in patients receiving adriamycin-cyclophosphamide-based chemotherapy. Support Care Cancer 19:807–813
Watcha MF, White PF (1992) Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology 77:162–184
Molassiotis A, Yam BM, Yung H, Chan FY, Mok TS (2002) Pretreatment factors predicting the development of postchemotherapy nausea and vomiting in Chinese breast cancer patients. Support Care Cancer 10:139–145
Roscoe JA, Bushunow P, Morrow GR et al (2004) Patient expectation is a strong predictor of severe nausea after chemotherapy: a University of Rochester Community Clinical Oncology Program study of patients with breast carcinoma. Cancer 101:2701–2708
Molassiotis A, Lee PH, Burke TA et al (2016) Anticipatory nausea, risk factors, and its impact on chemotherapy-induced nausea and vomiting: results from the Pan European Emesis Registry study. J Pain Symptom Manag 51:987–993
Clemons M, Bouganim N, Smith S et al (2016) Risk model-guided antiemetic prophylaxis vs physician’s choice in patients receiving chemotherapy for early-stage breast cancer: a Randomized Clinical Trial. JAMA Oncol 2:225–231
Molassiotis A, Stamataki Z, Kontopantelis E (2013) Development and preliminary validation of a risk prediction model for chemotherapy-related nausea and vomiting. Support Care Cancer 21:2759–2767
Bouganim N, Dranitsaris G, Hopkins S et al (2012) Prospective validation of risk prediction indexes for acute and delayed chemotherapy-induced nausea and vomiting. Curr Oncol 19:e414–e421
Dranitsaris G, Molassiotis A, Clemons M et al (2017) The development of a prediction tool to identify cancer patients at high risk for chemotherapy-induced nausea and vomiting. Ann Oncol 28:1260–1267
Thavorn K, Coyle D, Hoch JS et al (2017) A cost-utility analysis of risk model-guided versus physician’s choice antiemetic prophylaxis in patients receiving chemotherapy for early-stage breast cancer: a net benefit regression approach. Support Care Cancer 25:2505–2513
National Institutes of Health. A randomized trial of individualised care versus standard care for breast cancer patients at high risk for chemotherapy-induced nausea and vomiting. The ILIAD Study. Available at: https://clinicaltrials.gov/show/NCT02861859. Accessed 17 Jun 2017
Acknowledgements
The author would like to thank Tristin Abair, PhD, for her assistance in drafting the manuscript, and Trudy Grenon Stoddert, ELS, for her editorial assistance and assistance with preparing the manuscript for submission.
Financial support
This educational activity is supported by grants from Merck and Co, Inc. and TESARO, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Clemons, M. Guidelines versus individualized care for the management of CINV. Support Care Cancer 26 (Suppl 1), 11–17 (2018). https://doi.org/10.1007/s00520-018-4115-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4115-3